
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 20PS
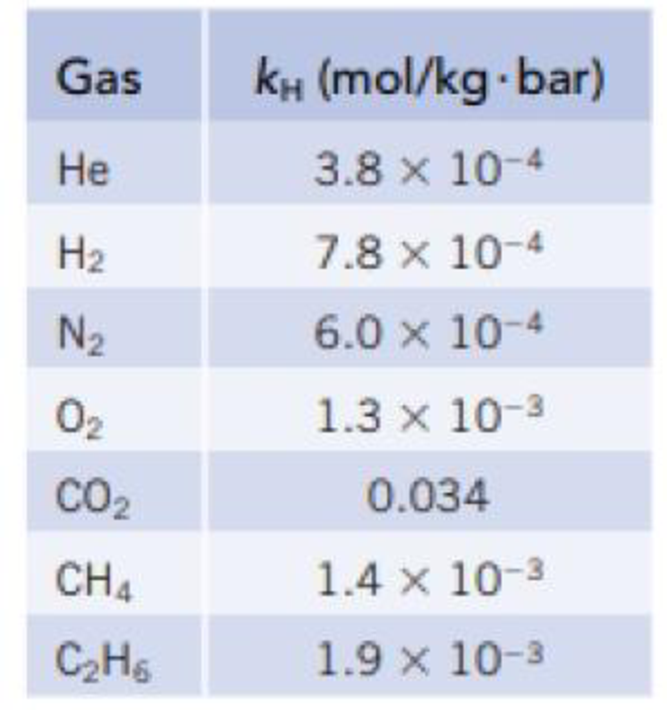
The Henry’s law constant for O2 in water at 25 ° is given in Table 13.2. Which of the following is a reasonable constant when the temperature is 50 °C? Explain the reason for your choice.
- (a) 6.7 × 10−4 mol/kg · bar
- (b) 2.6 × 10−3 mol/kg · bar
- (c) 1.3 × 10−3 mol/kg · bar
- (d) 6.4 × 10−3 mol/kg · bar
TABLE 13.2
Henry’s Law Constants for Gases in Water (25 °C)*
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the electron-domain geometry around thecentral B in BARF?
What is the Solubility of Oxygen O2 , in water ( in units of mL/1000g H2O)?
The freezing point of a 0.10 m solution of mercury(I) nitrate is approximately -0.27 celsius (Assuming that the person who prepared the solution thought it was HgNO3) Why do these data suggest that the formula of the mercury(I) ion is Hg2^2+ ? Assume the molecular formula for mercury(I) nitrate is HgNO3, calculate the freezing point based on this assumption.
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Ch. 13.1 - (a) If you dissolve 10.0 g (about one heaping...Ch. 13.2 - Use the data in Table 13.1 to calculate the...Ch. 13.3 - Prob. 13.3CYUCh. 13.4 - Assume you dissolve 10.0 g of sucrose (C12H22O11)...Ch. 13.4 - What quantity of ethylene glycol, HOCH2CH2OH, must...Ch. 13.4 - In the northern United States, summer cottages are...Ch. 13.4 - Bradykinin is a small peptide (9 amino acids; 1060...Ch. 13.4 - An aluminum-containing compound has the empirical...Ch. 13.4 - A 1.40-g sample of polyethylene, a common plastic,...Ch. 13.4 - Calculate the freezing point of 525 g of water...
Ch. 13.5 - The blue line on the diagram illustrates the...Ch. 13.5 - How many theoretical plates are required to...Ch. 13.5 - Prob. 1.3ACPCh. 13.5 - The vapor pressure of pure heptane is 361.5 mm Hg...Ch. 13.5 - If the headspace of a soda is 25 mL and the...Ch. 13.5 - Prob. 2.2ACPCh. 13.5 - Prob. 2.3ACPCh. 13.5 - Prob. 2.4ACPCh. 13.5 - Prob. 3.1ACPCh. 13.5 - Prob. 3.2ACPCh. 13.5 - Prob. 3.3ACPCh. 13 - You dissolve 2.56 g of succinic acid, C2H4(CO2H)2,...Ch. 13 - You dissolve 45.0 g of camphor, C10H16O, in 425 mL...Ch. 13 - Prob. 3PSCh. 13 - Prob. 4PSCh. 13 - Prob. 5PSCh. 13 - Prob. 6PSCh. 13 - Prob. 7PSCh. 13 - Prob. 8PSCh. 13 - Hydrochloric acid is sold as a concentrated...Ch. 13 - Concentrated sulfuric acid has a density of 1.84...Ch. 13 - The average lithium ion concentration in seawater...Ch. 13 - Silver ion has an average concentration of 28 ppb...Ch. 13 - Which pairs of liquids will be miscible? (a) H2O...Ch. 13 - Acetone, CH3COCH3, is quite soluble in water....Ch. 13 - Prob. 15PSCh. 13 - Use the following data to calculate the enthalpy...Ch. 13 - You make a saturated solution of NaCl at 25 C. No...Ch. 13 - Some lithium chloride, LiCl, is dissolved in 100...Ch. 13 - Prob. 19PSCh. 13 - The Henrys law constant for O2 in water at 25 is...Ch. 13 - An unopened soda can has an aqueous CO2...Ch. 13 - Hydrogen gas has a Henrys law constant of 7.8 104...Ch. 13 - A sealed flask contains water and oxygen gas at 25...Ch. 13 - Butane, C4H10, has been suggested as the...Ch. 13 - A 35.0-g sample of ethylene glycol, HOCH2CH2OH, is...Ch. 13 - Urea, (NH2)2CO, which is widely used in...Ch. 13 - Pure ethylene glycol, HOCH2CH2OH, is added 2.00 kg...Ch. 13 - Pure iodine (105 g) is dissolved in 325 g of CCl4...Ch. 13 - Prob. 29PSCh. 13 - What is the boiling point of a solution composed...Ch. 13 - Prob. 31PSCh. 13 - Prob. 32PSCh. 13 - Prob. 33PSCh. 13 - Some ethylene glycol, HOCH2CH2OH, is added to your...Ch. 13 - You dissolve 15.0 g of sucrose, C12H22O11, in a...Ch. 13 - A typical bottle of wine consists of an 11%...Ch. 13 - Prob. 37PSCh. 13 - Estimate the osmotic pressure of human blood at 37...Ch. 13 - An aqueous solution containing 1.00 g of bovine...Ch. 13 - Calculate the osmotic pressure of a 0.0120 M...Ch. 13 - You add 0.255 g of an orange, crystalline compound...Ch. 13 - Butylated hydroxyanisole (BHA) is used in...Ch. 13 - Benzyl acetate is one of the active components of...Ch. 13 - Anthracene, a hydrocarbon obtained from coal, has...Ch. 13 - An aqueous solution contains 0.180 g of an...Ch. 13 - Aluminon, an organic compound, is used as a...Ch. 13 - Prob. 47PSCh. 13 - To make homemade ice cream, you cool the milk and...Ch. 13 - List the following aqueous solutions in order of...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - When solutions of BaCl2 and Na2SO4 are mixed, the...Ch. 13 - The dispersed phase of a certain colloidal...Ch. 13 - Phenylcarbinol is used in nasal sprays as a...Ch. 13 - (a) Which aqueous solution is expected to have the...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - Prob. 56GQCh. 13 - Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a...Ch. 13 - A 10.7 m solution of NaOH has a density of 1.33...Ch. 13 - Concentrated aqueous ammonia has a molarity of...Ch. 13 - Prob. 60GQCh. 13 - If you want a solution that is 0.100 m in ions,...Ch. 13 - Consider the following aqueous solutions: (i) 0.20...Ch. 13 - (a) Which solution is expected to have the higher...Ch. 13 - The solubility of NaCl in water at 100 C is 39.1...Ch. 13 - Instead of using NaCl to melt the ice on your...Ch. 13 - The smell of ripe raspberries is due to...Ch. 13 - Hexachlorophene has been used in germicidal soap....Ch. 13 - The solubility of ammonium formate, NH4CHO2, in...Ch. 13 - How much N2 can dissolve in water at 25 C if the...Ch. 13 - Cigars are best stored in a humidor at 18 C and...Ch. 13 - An aqueous solution containing 10.0 g of starch...Ch. 13 - Prob. 72GQCh. 13 - Calculate the enthalpies of solution for Li2SO4...Ch. 13 - Water at 25 C has a density of 0.997 g/cm3....Ch. 13 - If a volatile solute is added to a volatile...Ch. 13 - A solution is made by adding 50.0 mL of ethanol...Ch. 13 - A 2.0% (by mass) aqueous solution of novocainium...Ch. 13 - A solution is 4.00% (by mass) maltose and 96.00%...Ch. 13 - The following table lists the concentrations of...Ch. 13 - A tree is 10.0 m tall. (a) What must be the total...Ch. 13 - Prob. 81GQCh. 13 - A compound is known to be a potassium halide, KX....Ch. 13 - Prob. 85GQCh. 13 - If one is very careful, it is possible to float a...Ch. 13 - A solution of benzoic acid in benzene has a...Ch. 13 - You dissolve 5.0 mg of iodine, I2, in 25 mL of...Ch. 13 - Prob. 89ILCh. 13 - In a police forensics lab, you examine a package...Ch. 13 - An organic compound contains carbon (71.17%),...Ch. 13 - Prob. 92ILCh. 13 - When sails of Mg2+, Ca2+, and Be2+ are placed in...Ch. 13 - Explain why a cucumber shrivels up when it is...Ch. 13 - Prob. 95SCQCh. 13 - A 100.-gram sample of sodium chloride (NaCl) is...Ch. 13 - Prob. 97SCQCh. 13 - Prob. 98SCQCh. 13 - Starch contains CC, CH, CO, and OH bonds....Ch. 13 - Prob. 100SCQCh. 13 - You have two aqueous solutions separated by a...Ch. 13 - Prob. 102SCQCh. 13 - Sodium chloride (NaCl) is commonly used to melt...Ch. 13 - Prob. 105SCQCh. 13 - Prob. 106SCQCh. 13 - Prob. 107SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6-70 In winter, after a snowstorm, salt (NaCI) is spread to melt the ice on roads. How many grams of salt per 1000. g of ice is needed to make it liquid at-5°C?arrow_forwardAn aqueous solution of LiX is prepared by dissolving 3.58 g of the electrolyte in 283 mL of H2O(d=1.00g/mL). The solution freezes at -1.81C. What is X-? (Assume complete dissociation of LiX to Li+ and X-.arrow_forward6-93 Two bottles of water are carbonated, with CO2 gas being added, under 2 atm pressure and then capped. One bottle is stored at room temperature; the other is stored in the refrigerator. When the bottle stored at room temperature is opened, large bubbles escape, along with a third of the water. The bottle stored in the refrigerator is opened without frothing or bubbles escaping. Explain.arrow_forward
- The volume required from (12N) HCl to prepare dilute solution(0.6 N), (100ml) isarrow_forwardA series of anions is shown below: The anion on the far right is called “BARF” by chemists, asits common abbreviation sounds similar to this word.(a) What is the central atom and the number of electronpairdomains around the central atom in each of theseanions?(b) What is the electron-domain geometry around thecentral B in BARF?(c) Which, if any, of these anions has an expanded octetaround its central atom?(d) Tetrabutylammonium, (CH3CH2CH2CH2)4N+ is abulky cation. Which anion, when paired with thetetrabutylammonium cation, would lead to a saltthat will be most soluble in nonpolar solvents?arrow_forwardThe Henry’s Law constant for N2 in water is 6.41x10^-4 mol/L•atm at 25°C. Calculate the concentration of N2 in water if the partial pressure of N2 is 3.70 atm?arrow_forward
- What pressure of carbon dioxide is required to keep the carbon dioxide concentration in a bottle of club soda at 0.12M at 25 degrees celcius?arrow_forwardA 18.9% by mass MnCl3 solution is prepared in water. What is the vapor pressure, in mmHg, at 75^oC?arrow_forward1.Identify the principal type of solute-solvent interaction in a solution of HF in CH3OH. answer choices: ion‑dipole interactions , dispersion forces, dipole‑dipole interactions, or hydrogen bondingarrow_forward
- The Henry’s law constant for O2 is 1.3 × 10−3 M/atm at 25 °C. What mass of oxygen would be dissolved in a 40-L aquarium at 25 °C, assuming an atmospheric pressure of 1.00 atm, and that the partial pressure of O2 is 0.21atm?arrow_forwardHow many grams of NiCl2 * 6H2O will be used to prepare a 0.0350 M, 500.0 mL of NiCl2 solution?arrow_forwardThe Henry's law constant for helium gas in water at 30 celsius is 3.70 x 10-4 M/atm. When the partial pressure of helium above a sample of water is 0.430atm, the concentration of helium in the water is __ M.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY