
Concept explainers
The following table lists the concentrations of the principal ions in seawater:
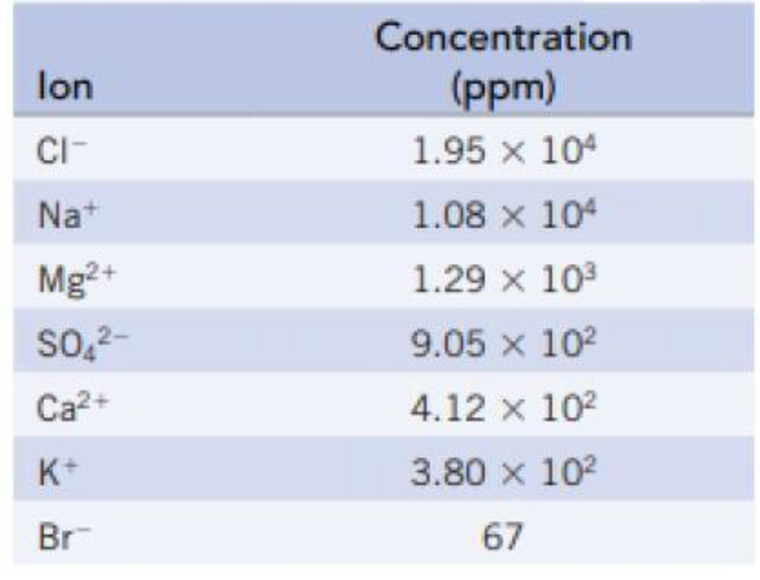
- (a) Calculate the freezing point of seawater.
- (b) Calculate the osmotic pressure of seawater at 25 °C. What is the minimum pressure needed to purify seawater by reverse osmosis?
(a)
Interpretation: The freezing point of seawater has to be determined.
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Freezing point depression: The freezing point of the solution varies with the solute concentration.
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
Freezing point of seawater is
Explanation of Solution
Given,
Molal freezing point depression constant of water is
The value
Hence, the concentration given in ppm can be taken as the mass of each of the ions on
The number of moles of any substance can be determined using the equation
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
So the total moles of principle ions in
Molality of seawater is,
Depression in freezing point is,
Therefore,
Freezing point of seawater is,
Freezing point of seawater is
(b)
Interpretation: The osmotic pressure of seawater at
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Osmotic pressure: The pressure created by the column of solution for the system at equilibrium is a measure of the osmotic pressure and is calculated by using the equation,
where,
c is the molar concentration
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
The osmotic pressure of seawater at
Explanation of Solution
Given,
The molarity of the solute is
The osmotic pressure of seawater is,
The osmotic pressure of seawater at
To purify the seawater using reverse osmosis method, there should be a minimum pressure of
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Chemistry
Introduction to Chemistry
Elementary Principles of Chemical Processes, Binder Ready Version
- A 1.00 mol/kg aqueous sulfuric acid solution, H2SO4,freezes at 4.04 C. Calculate i, the vant Hoff factor,for sulfuric acid in this solution.arrow_forward6-111 As noted in Section 6-8C, the amount of external pressure that must be applied to a more concentrated solution to stop the passage of solvent molecules across a semipermeable membrane is known as the osmotic pressure The osmotic pressure obeys a law similar in form to the ideal gas law (discussed in Section 5-4), where Substituting for pressure and solving for osmotic pressures gives the following equation: RT MRT, where M is the concentration or molarity of the solution. (a) Determine the osmotic pressure at 25°C of a 0.0020 M sucrose (C12H22O11) solution. (b) Seawater contains 3.4 g of salts for every liter of solution. Assuming the solute consists entirely of NaCl (and complete dissociation of the NaCI salt), calculate the osmotic pressure of seawater at 25°C. (c) The average osmotic pressure of blood is 7.7 atm at 25°C. What concentration of glucose (C6H12O6) will be isotonic with blood? (d) Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210. mL of solution has an osmotic pressure of 0.953 torr at 25°C. What is the molar mass of lysozyme? (e) The osmotic pressure of an aqueous solution of a certain protein was measured in order to determine the protein's molar mass. The solution contained 3.50 mg of protein dissolved in sufficient water to form 5.00 mL of solution. The osmotic pressure of the solution at 25°C was found to be 1.54 torr. Calculate the molar mass of the protein.arrow_forwardWhat is the freezing point and normal boiling point of a solution made by adding 39 mL of acetone, C3H6O, to 225 mL of water? The densities of acetone and water are 0.790 g/cm3 and 1.00 g/cm3, respectively.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





