
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 60AP
For the network in Figure P18.60, show that the resistance between points a and b is
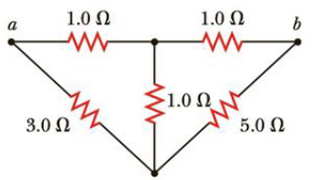
Figure P18.60
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 18 Solutions
College Physics
Ch. 18.1 - True or False: While discharging, the terminal...Ch. 18.1 - Why does a battery get warm while in use?Ch. 18.2 - In Figure 18.5, the current is measured with the...Ch. 18.2 - The circuit in Figure 18.5 consists of two...Ch. 18.3 - In Figure 18.8, the current is measured with the...Ch. 18.3 - When the switch is open in Figure 18.8, power Po...Ch. 18.3 - Suppose you have three identical lightbulbs, some...Ch. 18.3 - If the lightbulbs in Quick Quiz 18.7 are connected...Ch. 18.5 - The switch is closed in Figure 18.20. After a long...Ch. 18 - Choose the words that make each statement correct....
Ch. 18 - Given three lightbulbs and a battery, sketch as...Ch. 18 - Suppose the energy transferred to a dead battery...Ch. 18 - A short circuit is a circuit containing a path of...Ch. 18 - Electric current I enters a node with three...Ch. 18 - If electrical power is transmitted over long...Ch. 18 - The following statements are related to household...Ch. 18 - Two sets of Christmas lights are available. For...Ch. 18 - Why is it possible for a bird to sit on a...Ch. 18 - An uncharged series RC circuit is to be connected...Ch. 18 - Suppose a parachutist lands on a high-voltage wire...Ch. 18 - A ski resort consists of a few chairlifts and...Ch. 18 - Embodied in Kirchhoffs rules are two conservation...Ch. 18 - Why is it dangerous to turn on a light when you...Ch. 18 - A battery haring an emf of 9.00 V delivers 117 mA...Ch. 18 - Prob. 2PCh. 18 - A battery with an emf of 12.0 V has a terminal...Ch. 18 - A battery with a 0.100- internal resistance...Ch. 18 - Two resistors, R1 and R2 are connected in series....Ch. 18 - Three 9.0- resistors are connected in series with...Ch. 18 - (a) Find the equivalent resistance between points...Ch. 18 - Consider the combination of resistors shown in...Ch. 18 - Prob. 9PCh. 18 - Consider the circuit shown in Figure P18.10. (a)...Ch. 18 - Consider the circuit shown in Figure P18.11. Find...Ch. 18 - Four resistors are connected to a battery as shown...Ch. 18 - The resistance between terminals a and b in Figure...Ch. 18 - A battery with = 6.00 V and no internal...Ch. 18 - Find the current in the 12- resistor in Figure...Ch. 18 - (a) Is it possible to reduce the circuit shown in...Ch. 18 - (a) You need a 45- resistor, but the stockroom has...Ch. 18 - (a) Find the current in each resistor of Figure...Ch. 18 - Figure P18.19 shows a Wheatstone bridge, a circuit...Ch. 18 - For the circuit shown in Figure P18.20, calculate...Ch. 18 - Taking R = 1.00 k and = 250 V in Figure P18.21,...Ch. 18 - In the circuit of Figure P18.22, the current I1 is...Ch. 18 - In the circuit of Figure P18.23, determine (a) the...Ch. 18 - Four resistors are connected to a battery with a...Ch. 18 - Using Kirchhoffs rules (a) find the current in...Ch. 18 - Figure P18.26 shows a voltage divider, a circuit...Ch. 18 - (a) Can the circuit shown in Figure P18.27 be...Ch. 18 - A dead battery is charged by connecting it to the...Ch. 18 - (a) Can the circuit shown in Figure P18.29 be...Ch. 18 - For the circuit shown in Figure P18.30, use...Ch. 18 - Find the potential difference across each resistor...Ch. 18 - Show that = RC has units of time.Ch. 18 - Consider the series RC circuit shown in Figure...Ch. 18 - An uncharged capacitor and a resistor are...Ch. 18 - Consider a series RC circuit as in Figure P18.35...Ch. 18 - The RC charging circuit in a camera flash unit has...Ch. 18 - Figure P18.37 shows a simplified model of a...Ch. 18 - The capacitor in Figure P18.35 is uncharged for t ...Ch. 18 - What minimum number of 75-W light bulbs must be...Ch. 18 - A 1 150-W toaster and an 825-W microwave oven are...Ch. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Assume a length of axon membrane of about 0.10 m...Ch. 18 - Consider the model of the axon as a capacitor from...Ch. 18 - Prob. 45PCh. 18 - How many different resistance values can be...Ch. 18 - (a) Calculate the potential difference between...Ch. 18 - For the circuit shown in Figure P18.48, the...Ch. 18 - Figure P18.49 shows separate series and parallel...Ch. 18 - Three 60.0-W, 120-V lightbulbs are connected...Ch. 18 - When two unknown resistors are connected in series...Ch. 18 - The circuit in Figure P18.52a consists of three...Ch. 18 - A circuit consists of three identical lamps, each...Ch. 18 - The resistance between points a and b in Figure...Ch. 18 - The circuit in Figure P18.55 has been connected...Ch. 18 - Prob. 56APCh. 18 - The student engineer of a campus radio station...Ch. 18 - The resistor R in Figure P18.58 dissipates 20 W of...Ch. 18 - A voltage V is applied to a series configuration...Ch. 18 - For the network in Figure P18.60, show that the...Ch. 18 - A battery with an internal resistance of 10.0 ...Ch. 18 - The circuit in Figure P18.62 contains two...Ch. 18 - An electric eel generates electric currents...Ch. 18 - In Figure P18.64, R1 = 0.100 , R2 = 1.00 , and R3...Ch. 18 - What are the expected readings of the ammeter and...Ch. 18 - Consider the two arrangements of batteries and...Ch. 18 - The given pair of capacitors in Figure P18.67 is...Ch. 18 - 2.00-nF capacitor with an initial charge of 5.10 C...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (a) Can the circuit shown in Figure P18.29 be reduced to a single resistor connected to the batteries? Explain. (b) Find the magnitude of the current and its direction in each resistor. Figure P18.29arrow_forwardCalculate the equivalent resistance between points P and Q of the electrical network shown in Figure P29.80.arrow_forwardA battery is used to charge a capacitor through a resistor as shown in Figure P27.44. Show that half the energy supplied by the battery appears as internal energy in the resistor and half is stored in the capacitor. Figure P27.44arrow_forward
- Electric current I enters a node with three resistors connected in parallel (Fig. CQ18.5). Which one of the following is correct? (a) I1 = I and I2 = I3 = 0. (b) I2 I1 and I2 I3. (c) V1 V2 V3 (d) I1 I2 I3 0. Figure CQ18.5arrow_forwardElectric current I enters a node with three resistors connected in parallel (Fig. CQ18.5). Which one of the following is correct? (a) I1 = I and I2 = I3 = 0. (b) I2 I1 and I2 I3. (c) V1 V2 V3 (d) I1 I2 I3 0. Figure CQ18.5arrow_forwardThe circuit in Figure P18.55 has been connected for several seconds. Find the current (a) in the 4.00-V battery,(b) in the 3.00- resistor,(c)in the 8.00-V battery, and (d)in the 3.00-V battery.(e)Find the charge on the capacitor.arrow_forward
- Three 60.0-W, 120-V lightbulbs are connected across a 120-V power source, as shown in Figure P18.50. Find (a) the total power delivered to the three bulbs and (b) the potential difference across each. Assume the resistance of each bulb is constant (even though, in reality, the resistance increases markedly with current). Figure P18.50arrow_forwardConsider the combination of resistors shown in Figure P18.8. (a) Find the equivalent resistance between point a and b. (b) If a voltage of 35.0 V is applied between points a and b, find the current in each resistor. Figure P18.8arrow_forwardThe ammeter shown in Figure P21.45 reads 2.00 A. Find I1, I2, and . Figure P21.45arrow_forward
- Figure P18.19 shows a Wheatstone bridge, a circuit used to precisely measure an unknown resistance R by varying Rvar until the ammeter reads zero current and the bridge is said to be balanced. If the bridge is balanced with Rvar = 9.00 , find (a) the value of the unknown resistance Rand (b) the current in the 1.00 resistor. (Hint: With the bridge balanced, the wire through the ammeter can effectively be removed from the circuit, leaving two pairs of resistors in parallel.) Figure Pl8.19arrow_forwardConsider the circuit shown in Figure P21.39. Find (a) the current in the 20.0- resistor and (b) the potential difference between points a and b. Figure P21.39arrow_forwardA 12.0-V emf automobile battery has a terminal voltage of 16.0 V when being charged by a current of 10.0 A. (a) What is the battery’s internal resistance? (b) What power is dissipated inside the battery? (c) At what rate (in °C/min ) will its temperature increase if its mass is 20.0 kg and it has a specific heat of 0.300 kcal/kg • °C, assuming no heat escapes?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
How To Solve Any Resistors In Series and Parallel Combination Circuit Problems in Physics; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=eFlJy0cPbsY;License: Standard YouTube License, CC-BY