
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 29PS
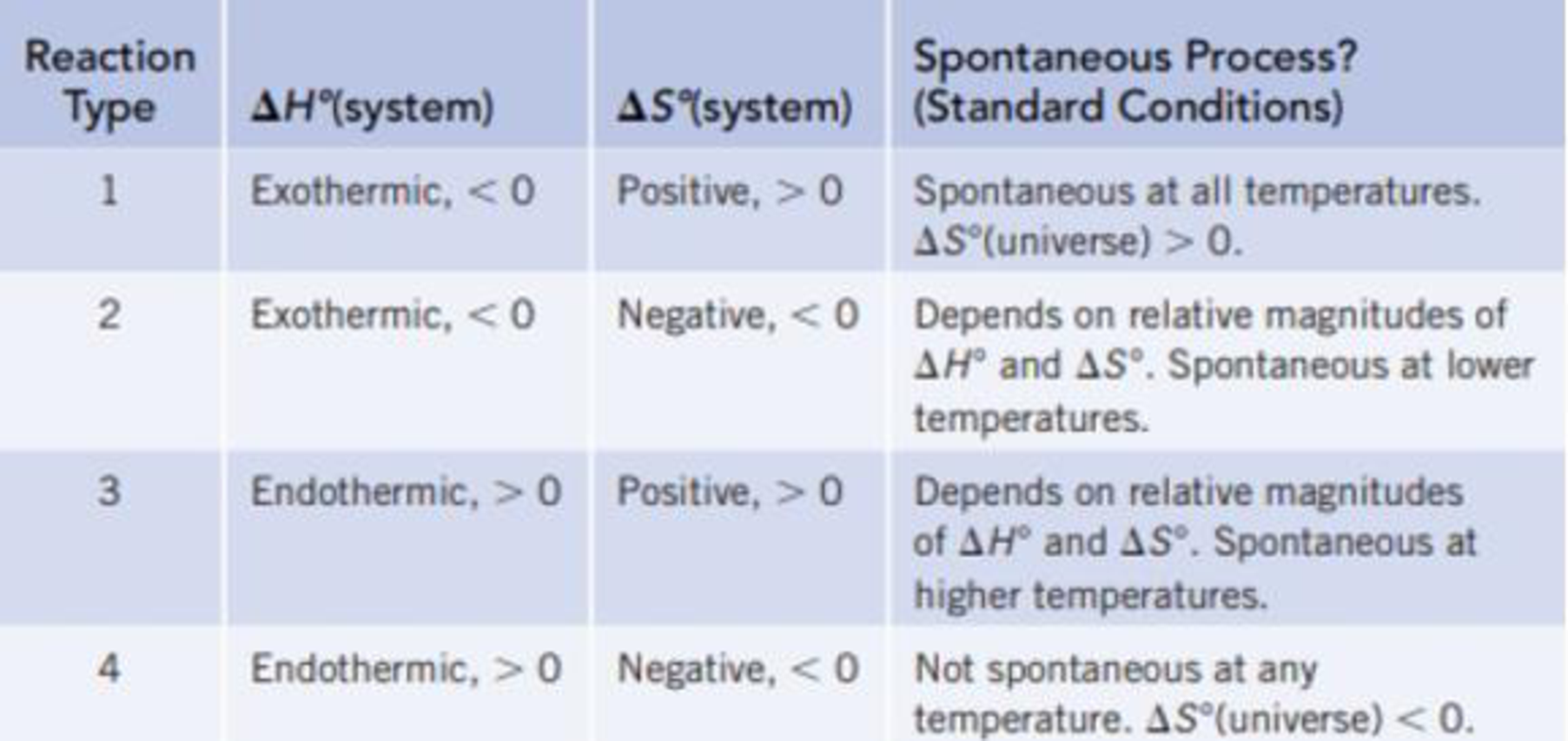
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.
- (a) Fe2O3(s) + 2 Al(s) → 2 Fe(s) + Al2O3(s)
ΔrH° = −851.5 kj/mol-rxn
ΔrS° = −375.2 J/K · mol-rxn
- (b) N2(g) + 2 O2(g) → 2 NO2(g)
ΔrH° = 66.2 kJ/mol-rxn
ΔrS° = −121.6 J/K · mol-rxn
TABLE 18.1 Predicting Whether a Reaction Will Be Spontaneous Under Standard Conditions
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Chemistry & Chemical Reactivity
Ch. 18.3 - Predict which substance in each pair has the...Ch. 18.3 - Prob. 18.2CYUCh. 18.4 - Based on rH and rS, predict the spontaneity of the...Ch. 18.6 - Prob. 18.4CYUCh. 18.6 - Prob. 18.5CYUCh. 18.6 - Oxygen was first prepared by Joseph Priestley...Ch. 18.6 - Prob. 18.7CYUCh. 18.6 - Prob. 18.8CYUCh. 18.6 - Prob. 18.9CYUCh. 18.7 - Consider the hydrolysis reactions of creatine...
Ch. 18.7 - Prob. 1.2ACPCh. 18.7 - The decomposition of diamond to graphite...Ch. 18.7 - It has been demonstrated that buckminsterfullerene...Ch. 18 - Solid NH4NO3 is placed in a beaker containing...Ch. 18 - Acetic acid, a weak acid, was added to a beaker...Ch. 18 - Identify the following processes as either...Ch. 18 - Identify the following processes as either...Ch. 18 - Prob. 5PSCh. 18 - Predict whether each of the following processes...Ch. 18 - Indicate which of the following processes are...Ch. 18 - Prob. 8PSCh. 18 - Prob. 9PSCh. 18 - Prob. 10PSCh. 18 - Prob. 11PSCh. 18 - Calculate the entropy change that occurs when 1.00...Ch. 18 - Prob. 13PSCh. 18 - Calculate the change in entropy of a system with...Ch. 18 - The third law of thermodynamics says that a...Ch. 18 - Identify trends in S values: (a) For the halogens:...Ch. 18 - Which substance has the higher entropy? (a) dry...Ch. 18 - Which substance has the higher entropy? (a) a...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Is the reaction Si(s) + 2 Cl2(g) SiCl4(g)...Ch. 18 - Is the reaction Si(s) + 2 H2(g) SiH4(g)...Ch. 18 - Calculate S(universe) for the decomposition of 1...Ch. 18 - Calculate S(universe) for the formation of 1 mol...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - For the reaction BaCO3(s) BaO(s) + CO2(g), rG =...Ch. 18 - For the reaction TiCl2(s) + Cl2(g) TiCl4(), rG =...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Heating some metal carbonates, among them...Ch. 18 - Calculate rH and rS for the reaction of tin(IV)...Ch. 18 - The ionization constant, Ka, for acetic acid is...Ch. 18 - Prob. 44PSCh. 18 - The standard free energy change, rG, for the...Ch. 18 - Prob. 46PSCh. 18 - Calculate rG at 25 C for the formation of 1.00 mol...Ch. 18 - Prob. 48PSCh. 18 - Prob. 49PSCh. 18 - Prob. 50PSCh. 18 - Compare the compounds in each set below and decide...Ch. 18 - Using standard entropy values, calculate rS for...Ch. 18 - About 5 billion kilograms of benzene, C6H6, are...Ch. 18 - Hydrogenation, the addition of hydrogen to an...Ch. 18 - Is the combustion of ethane, C2H6, product-favored...Ch. 18 - Prob. 56GQCh. 18 - When vapors from hydrochloric acid and aqueous...Ch. 18 - Calculate S(system), S(surroundings), and...Ch. 18 - Methanol is now widely used as a fuel in race...Ch. 18 - The enthalpy of vaporization of liquid diethyl...Ch. 18 - Calculate the entropy change, rS, for the...Ch. 18 - Using thermodynamic data, estimate the normal...Ch. 18 - Prob. 63GQCh. 18 - When calcium carbonate is heated strongly, CO2 gas...Ch. 18 - Sodium reacts violently with water according to...Ch. 18 - Yeast can produce ethanol by the fermentation of...Ch. 18 - Elemental boron, in the form of thin fibers, can...Ch. 18 - Prob. 68GQCh. 18 - Prob. 69GQCh. 18 - Estimate the boiling point of water in Denver,...Ch. 18 - The equilibrium constant for the butane ...Ch. 18 - A crucial reaction for the production of synthetic...Ch. 18 - Calculate rG for the decomposition of sulfur...Ch. 18 - Prob. 74GQCh. 18 - A cave in Mexico was recently discovered to have...Ch. 18 - Wet limestone is used to scrub SO2 gas from the...Ch. 18 - Sulfur undergoes a phase transition between 80 and...Ch. 18 - Calculate the entropy change for dissolving HCl...Ch. 18 - Some metal oxides can be decomposed to the metal...Ch. 18 - Prob. 80ILCh. 18 - Prob. 81ILCh. 18 - Prob. 82ILCh. 18 - Titanium(IV) oxide is converted to titanium...Ch. 18 - Cisplatin [cis-diamminedichloroplatinum(II)] is a...Ch. 18 - Prob. 85ILCh. 18 - Explain why each of the following statements is...Ch. 18 - Decide whether each of the following statements is...Ch. 18 - Under what conditions is the entropy of a pure...Ch. 18 - Prob. 89SCQCh. 18 - Consider the formation of NO(g) from its elements....Ch. 18 - Prob. 91SCQCh. 18 - The normal melting point of benzene, C6H6, is 5.5...Ch. 18 - Prob. 93SCQCh. 18 - For each of the following processes, predict the...Ch. 18 - Heater Meals are food packages that contain their...Ch. 18 - Prob. 96SCQCh. 18 - Prob. 97SCQCh. 18 - Prob. 98SCQCh. 18 - Iodine, I2, dissolves readily in carbon...Ch. 18 - Prob. 100SCQCh. 18 - Prob. 101SCQCh. 18 - Prob. 102SCQCh. 18 - Prob. 103SCQCh. 18 - Prob. 104SCQCh. 18 - The Haber-Bosch process for the production of...Ch. 18 - Prob. 106SCQCh. 18 - Prob. 107SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cobalt(II) chloride hexahydrate, CoCl26H2O, is a bright pink compound, but in the presence of very dry air it loses water vapor to the air to produce the light blue anhydrous salt CoCl2. Calculate the standard free-energy change for the reaction at 25C: CoCl26H2O(s)CoCl2(s)+6H2O(g) Here are some thermodynamic data at 25C: What is the partial pressure of water vapor in equilibrium with the anhydrous salt and the hexahydrate at 25C? (Give the value in mmHg.) What is the relative humidity of air that has this partial pressure of water? The relative humidity of a sample of air is Relativehumidity=partialpressureofH2O(g)inairvaporpressureofwater100 What do you expect to happen to the equilibrium partial pressure over the hexahydrate as the temperature is raised? Explain.arrow_forwardSilver carbonate, Ag2CO3, is a light yellow compound that decomposes when heated to give silver oxide and carbon dioxide: Ag2CO3(s)Ag2O(s)+CO2(g) A researcher measured the partial pressure of carbon dioxide over a sample of silver carbonate at 220C and found that it was 1.37 atm. Calculate the partial pressure of carbon dioxide at 25C. The standard enthalpies of formation of silver carbonate and silver oxide at 25C are 505.9 kJ/mol and 31.05 kJ/mol, respectively. Make any reasonable assumptions in your calculations. State the assumptions that you make, and note why you think they are reasonable.arrow_forwardThe free energy of formation of one mole of compound refers to a particular chemical equation. For each of the following, write that equation. a KBr(s) b CH3Cl(l) c H2S(g) d AsH3(g)arrow_forward
- The free energy of formation of one mole of compound refers to a particular chemical equation. For each of the following, write that equation. a MgO(s) b COCl2(g) c CF4(g) d PCl5(g)arrow_forwardElemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 32 H2(g) B(s) + 3 HCl(g) Calculate rH, rS, and rG at 25 C for this reaction. Is the reaction predicted to be product-favored at equilibrium at 25 C? If so, is it enthalpy- or entropy-driven? [S for B(s) is 5.86 J/K mol.]arrow_forwarda Calculate K1, at 25C for phosphoric acid: H3PO4(aq)H+(aq)+H2PO4(aq) b Which thermodynamic factor is the most significant in accounting for the fact that phosphoric acid is a weak acid? Why ?arrow_forward
- According to a source, lithium peroxide (Li2O2) decomposes to lithium oxide (Li2O) and oxygen gas at about 195C. If the standard enthalpy change for this decomposition is 33.9 kJ/mol, what would you give as an estimate for the standard entropy change for this reaction? Explain.arrow_forwardFor the decomposition of formic acid, HCOOH(l)H2O(l)+CO(g) H = +29 kJ/mol at 25C. a Does the tendency of this reaction to proceed to a state of minimum energy favor the formation of water and carbon monoxide or formic acid? Explain. b Does the tendency of this reaction to proceed to a state of maximum entropy favor the formation of products or reactants? Explainarrow_forwardThe direct reaction of iron(III) oxide. Fe2O3, to give iron and oxygen gas is a nonspontaneous reaction; normally, iron combines with oxygen to give rust (the oxide). Yet we do change iron(III) oxide, as iron ore, into iron metal. How is this possible? Explain.arrow_forward
- Elemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 3/2 H2(g) B(s) + 3HCl(g) Calculate H, S, and G at 25 C for this reaction. Is the reaction predicted to be product favored at equilibrium at 25 C? If so, is it enthalpy driven or entropy driven?arrow_forwardClassify the following processes as exergonic or endergonic. Explain your answers. a.Any combustion process b.Perspiration evaporation from the skin c.Melted lead solidifying d.An explosive detonating e.An automobile being pushed up a slight hill from point of view of the automobilearrow_forwardPick the example with the highest entropy from each of the following sets. Explain your answers. a.Two opposing football teams just before the ball is snapped, two opposing football teams 1 second after the ball is snapped, two opposing football teams when the whistle is blown, ending the play b.A 10 copper/gold alloy, a 2 copper/gold alloy, pure gold c.A purse on which the strap just broke, a purse just hitting the ground, a purse on the ground with contents scattered d.Coins in a piggy bank, coins in piles containing the same type of coins, coins in stacks of the same type of coins e.A dozen loose pearls in a box, a dozen pearls randomly strung on a string, a dozen pearls strung on a string in order of decreasing sizearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY