
College Physics
1st Edition
ISBN: 9781938168000
Author: Paul Peter Urone, Roger Hinrichs
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 31, Problem 69PE
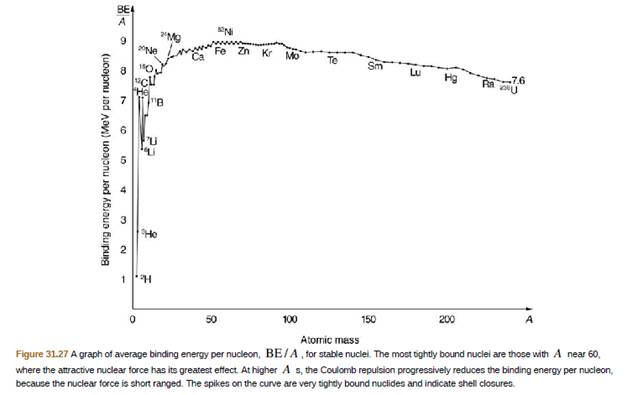
2H is a loosely hound isotope of hydrogen. Called deuterium or heavy hydrogen, it is stable but relatively rare−it is 0.015% of natural hydrogen. Note that deuterium has Z = N, which should tend to make it more tightly bound, but both are odd numbers. Calculate BE/A, the binding energy per nucleon, for 2H and compare it with the approximate value obtained from line graph in Figure 31.27.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 31 Solutions
College Physics
Ch. 31 - Suppose the range for 5.0 MeVa ray is known to be...Ch. 31 - What is the difference between (rays and...Ch. 31 - Ionizing radiation interacts with matter by...Ch. 31 - What characteristics of radioactivity show it to...Ch. 31 - What is the source of the energy emitted in...Ch. 31 - Consider Figure 31.3. If an electric field is...Ch. 31 - Explain how an (particle can have a larger range...Ch. 31 - Arrange the following according to their ability...Ch. 31 - Often, when people have to work around radioactive...Ch. 31 - Is it possible for light emitted by a scintillator...
Ch. 31 - The weak and strong nuclear forces are basic to...Ch. 31 - Define and make clear distinctions between the...Ch. 31 - What are isotopes? Why do different isotopes of...Ch. 31 - Star Trek fans have often heard the term...Ch. 31 - What conservation law requires an electron’s...Ch. 31 - Neutrinos are experimentally determined to have an...Ch. 31 - What do the three types of beta decay have in...Ch. 31 - In a 3109 yearold rock that originally contained...Ch. 31 - Does the number of radioactive nuclei in a sample...Ch. 31 - Radioactivity depends on the nucleus and not the...Ch. 31 - Explain how a bound system can have less mass than...Ch. 31 - Spontaneous radioactive decay occurs only when the...Ch. 31 - To obtain the most precise value of BE from the...Ch. 31 - How does the finite range of the nuclear force...Ch. 31 - Why is the number of neutrons greater than the...Ch. 31 - A physics student caught breaking conservation...Ch. 31 - When a nucleus (decays, does the (particle move...Ch. 31 - The energy of 30.0 eV is required to ionize a...Ch. 31 - A particle of ionizing radiation creates 4000 ion...Ch. 31 - (a) Repeat Exercise 31.2, and convert the energy...Ch. 31 - Suppose a particle of ionizing radiation deposits...Ch. 31 - Verify that a 2.31017kg mass of water at normal...Ch. 31 - Find the length of a side of a cube having a mass...Ch. 31 - What is the radius of an (particle?Ch. 31 - Find the radius of a 238Pu nucleus. 238Pu is a...Ch. 31 - (a) Calculate the radius of 58Ni, one of the most...Ch. 31 - The unified atomic mass unit is defined to be...Ch. 31 - What is the ratio of the velocity of a (particle...Ch. 31 - If a 1.50cmthick piece of lead can absorb 90.0% of...Ch. 31 - The detail observable using a probe is limited by...Ch. 31 - (a) Show that if you assume the average nucleus is...Ch. 31 - What is the radio of the velocity of a 5.00MeV...Ch. 31 - (a) What is the kinetic energy in MeV of a ray...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - decay producing 137Ba. The parent nuclide is a...Ch. 31 - ( decay producing 90Y. The parent nuclide is a...Ch. 31 - decay producing 228Ra. The parent nuclide is...Ch. 31 - decay producing 208Pb. The parent nuclide is in...Ch. 31 - When an electron and position annihilate, both...Ch. 31 - Confirm That charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - A rare decay mode has been observed in which 222Ra...Ch. 31 - (a) Write the complete a decay equation for 226Ra....Ch. 31 - (a) Write the complete a decay equation for 249Cf....Ch. 31 - (a) Write the complete decay equation for the...Ch. 31 - (a) Write the complete decay equation for 90Sr,...Ch. 31 - Calculate the energy released in the + decay of...Ch. 31 - (a) Write the complete + decay equation for llC....Ch. 31 - (a) Calculate the energy released in the a decay...Ch. 31 - (a) Write the complete reaction equation for...Ch. 31 - (a) Write the complete reaction equation for...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - 2H is a loosely hound isotope of hydrogen. Called...Ch. 31 - 56Feis among the most tightly bound of all...Ch. 31 - 209Bi is the heaviest stable nuclide, and its BE/A...Ch. 31 - (a) Calculate BE/A for 235U, the rarer of the two...Ch. 31 - (a) Calculate BE/A for 12C. Stable and relatively...Ch. 31 - The fact that BE/A is greatest for A near 60...Ch. 31 - The purpose of this problem is to show in three...Ch. 31 - Unreasonable Results A particle physicist...Ch. 31 - Derive an approximate relationship between the...Ch. 31 - Integrated Concepts A 2.00T magnetic ?eld is...Ch. 31 - (a) Write the decay equation for the decay of...Ch. 31 - Unreasonable Results The relatively scarce...Ch. 31 - Unreasonable Results A physicist scatters (rays...Ch. 31 - Unreasonable Results A frazzled theoretical...Ch. 31 - Construct Your Own Problem Consider the decay of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. A cyclist goes around a level, circular track at constant speed. Do you agree or disagree with the following...
College Physics: A Strategic Approach (3rd Edition)
If acceleration is proportional to the net force or is equal to net force.
Conceptual Physics (12th Edition)
(II) What is the magnitude of the acceleration of a speck of clay on the edge of a potter's wheel turning at 45...
Physics: Principles with Applications
TEST YOUR UNDERSTANDING OF SECTION 9.1 The figure shows a graph of ?z and ?z versus time for a particular rotat...
University Physics with Modern Physics (14th Edition)
The pV-diagram of the Carnot cycle.
Sears And Zemansky's University Physics With Modern Physics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 56 Fe is among the most tightly bound of all nuclides.It makes up more than 90% of natural iron. Note that 56 Fe has even numbers of protons and neutrons. Calculate the binding energy per nucleon for 6Fe and compare it with the approximate value obtained from the graph in Figure 10.7.arrow_forward(a) Find the total energy released in MeV in each carbon cycle (elaborated in the above problem) including the annihilation energy. (b) How does this compare with the protonproton cycle output?arrow_forwardis the heaviest stable nuclide, and its BEN is low compared with medium-mass nuclides. Calculate BEN for this nucleus and compare it with the approximate value obtained from the graph in Figure 10.7. fission of nuclei with mass numbers greater than that of Fe. are othermic processes.arrow_forward
- (a) Neutron activation of sodium, which is 100% 23Na, produces 24Na, which is used in some heart scans, as seen in Table 32.1. The equation for the reaction is 23Na+n24Na+ . Find its energy output, given the mass of 24Na is 23.990962 u. (b) What mass at 24Na produces the needed 5.0mCi activity, given its halflife is 15.0 h?arrow_forwardDerive an approximate relationship between the energy of (decay and halflife using the following data. It may be useful to graph the leg t1/2 against Ea to find some straightline relationship. Table 31.3 Energy and HalfLife for (Decay Nuclide E( (MeV) t1/2 216Ra 9.5 0.18 (s 194Po 7.0 0.7 s 240Cm 6.4 27 d 226Ra 4.91 1600 y 232Th 4.1 1.41010yarrow_forwardIn the following eight problems, write the complete decay equation for the given nuclide in the complete XZAN notation. Refer to the periodic table for values of Z. decay of 226Ra, another isotope in the decay series of 238U, FIrst recognized as a new element by the Curies. Poses special problems because its daughter is a radioactive noble gas. In the following four problems, identity the parent nuclide and write the complete decay equation in the XZAN notation. Refer to the periodic table for values of Z.arrow_forward
- (a) Calculate the radius of 58Ni, one of the most tightly bound stable nuclei. (b) What is the ratio of the radius of 58Ni to that at 258Ha, one of the largest nuclei ever made? Note that the radius of the largest nucleus is still much smaller than ?le size of an atom.arrow_forward(a) Calculate the number of grams of deuterium in an 80.000L swimming pool, given deuterium is 0.0150% of natural hydrogen. (b) Find the energy released in joules if this deuterium is fused via the reaction 2H+2H3He+n. (c) Could the neutrons be used to create more energy? (d) Discuss the amount of this type of energy in a swimming pool as compared to that in, say, a gallon of gasoline, also taking into consideration that water is far more abundant.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
