
The reaction
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
was studied at 904 °C, and the data in the table were collected.
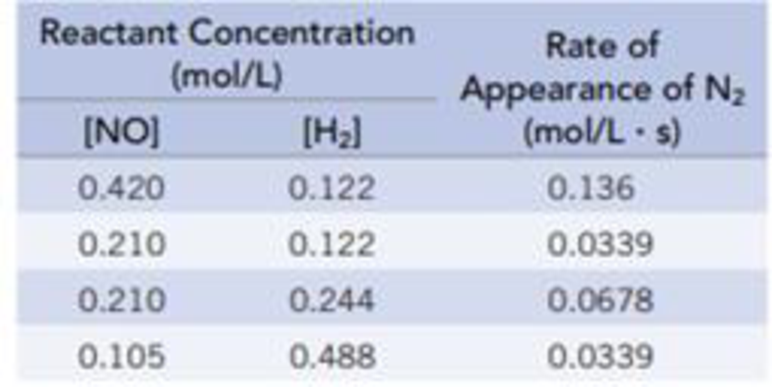
- (a) Determine the order of the reaction for each reactant.
- (b) Write the rate equation for the reaction.
- (c) Calculate the rate constant for the reaction.
- (d) Find the rate of appearance of N2 at the instant when [NO] = 0.350 mol/L and [H] = 0.205 mol/L.
(a)
Interpretation: The order of the reaction for each reactant has to be calculated.
Concept introduction:
Rate law or rate equation: The relationship between the reactant concentrations and reaction rate is expressed by an equation.
Order of a reaction: The order of a reaction with respect to a particular reactant is the exponent of its concentration term in the rate law expression, and the overall reaction order is the sum of the exponents on all concentration terms.
Rate constant, k: It is a proportionality constant that relates rate and concentration at a given temperature.
Answer to Problem 12PS
The order of
Explanation of Solution
The order of the reaction is calculated as,
In order to figure out the reaction equation the order of the reactants needed, which is calculated by comparing any two experiments where the concentration of
(b)
Interpretation: The rate of the reaction has to be written.
Concept introduction:
Rate law or rate equation: The relationship between the reactant concentrations and reaction rate is expressed by an equation.
Order of a reaction: The order of a reaction with respect to a particular reactant is the exponent of its concentration term in the rate law expression, and the overall reaction order is the sum of the exponents on all concentration terms.
Rate constant, k: It is a proportionality constant that relates rate and concentration at a given temperature.
Answer to Problem 12PS
The rate equation is
Explanation of Solution
The reaction rate:
Hence, Rate equation is
(c)
Interpretation: The order of the reaction for each reactant has to be calculated.
Concept introduction:
Rate law or rate equation: The relationship between the reactant concentrations and reaction rate is expressed by an equation.
Order of a reaction: The order of a reaction with respect to a particular reactant is the exponent of its concentration term in the rate law expression, and the overall reaction order is the sum of the exponents on all concentration terms.
Rate constant, k: It is a proportionality constant that relates rate and concentration at a given temperature.
Answer to Problem 12PS
The value of rate constant is
Explanation of Solution
The value of rate constant is calculated as,
The rate constant value is obtained as shown above. By substituting the any one of the concentrations of reactants and the initial rate into the reaction equation obtained at first. Hence, the value of rate constant is
(d)
Interpretation:
The rate of appearance of
Concept introduction:
Rate law or rate equation: The relationship between the reactant concentrations and reaction rate is expressed by an equation.
Order of a reaction: The order of a reaction with respect to a particular reactant is the exponent of its concentration term in the rate law expression, and the overall reaction order is the sum of the exponents on all concentration terms.
Rate constant, k: It is a proportionality constant that relates rate and concentration at a given temperature.
Answer to Problem 12PS
The rate when
Explanation of Solution
Calculate the reaction rate of any one experiment:
Calculate the rate of appearance of nitrogen:
The rate
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: The Central Science (13th Edition)
- At 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forwardSucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forwardThe following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,. Temperature (K) 3.5 x 10_i 298 2.2 x 10"4 308 6.8 X IO-4 318 3.1 x 10 1 328 Determine Etfor this reaction in kj/mol.arrow_forward
- Under certain conditions the decomposition of ammonia on a metal surface gives the following data: [NH3] (M) 1.0103 2.0103 3.0103 Rate (moI/L/h1) 1.5106 1.5106 1.5106 Determine the rate equation, the rate constant, and the overall order for this reaction.arrow_forwardOzone, O3, in the Earths upper atmosphere decomposes according to the equation 2 O3(g) 3 O2(g) The mechanism of the reaction is thought to proceed through an initial fast, reversible step followed by a slow, second step. Step 1: Fast, reversible O3(g) O2(g) + O(g) Step 2: Slow O3(g) + O(g) 2 O2(g) (a) Which of the steps is rate-determining? (b) Write the rate equation for the rate-determining steparrow_forwardThe initial rate ( [NO]/ t] of the reaction of nitrogen monoxide and oxygen NO(g) + 2O2(g) NO2(g) was measured for various initial concentrations of NO and O2 at 25 C. Determine the rate equation from these data. What is the value of the rate constant, k, and what are its units?arrow_forward
- For a reaction involving the decomposition of Z at a certain temperature, the following data are obtained: (a) What is the order of the reaction? (b) Write the rate expression for the decomposition of Z. (c) Calculate k for the decomposition at that temperature.arrow_forwardGive at least two physical properties that might be used to determine the rate of a reaction.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





