
Concept explainers
You titrate 25.0 mL of 0.10 M NH3 with 0.10 M HCl.
- (a) What is the pH of the NH3 solution before the titration begins?
- (b) What is the pH at the equivalence point?
- (c) What is the pH at the halfway point of the titration?
- (d) What indicator in Figure 17.11 could be used to detect the equivalence point?
- (e) Calculate the pH of the solution after adding 5.00, 15.0, 20.0, 22.0, and 30.0 mL of the acid. Combine this information with that in parts (a)–(c) and plot the titration curve.
(a)
Interpretation:
The value of
Concept introduction:
Titration is a quantitative method to determine the quantity of an acid or base in a solution. This method is used to determine the concentration an acid in the solution by titrating it against a base.
For strong acid-weak base titration, the
Calculation of
(1) The
(2) The
As the addition of
At the midpoint of the titration, the concentration of acid and its conjugate base is equal. Therefore
Substitute,
Therefore,
(3) The
At equivalence point, all the base will be neutralized, and there will be only
By using the value of
The relation between
Explanation of Solution
The
Given:
Refer to the Appendix I in the textbook for the value of
The value of
The initial concentration of
The initial concentration of
The volume of the solvent is
Therefore the volume of the solvent is
ICE table (1) gives the ionization reaction of
From the ICE table (1),
The concentration of
The concentration of ammonium ion produced after the reaction is
The concentration of
There is an approximation, that the value of
Therefore, Concentration of
The hydroxide ion concentration is calculated by the expression,
Substitute
Rearrange for
Therefore, the concentration of hydroxide ion is
Calculate the
Substitute
The relation between
Substitute the value of
The value of
(b)
Interpretation:
The value of
Concept introduction:
Titration is a quantitative method to determine the quantity of an acid or base in a solution. This method is used to determine the concentration an acid in the solution by titrating it against a base.
For strong acid-weak base titration, the
Calculation of
(1) The
(2) The
As the addition of
At the midpoint of the titration, the concentration of acid and its conjugate base is equal. Therefore
Substitute,
Therefore,
(3) The
At equivalence point, all the base will be neutralized, and there will be only
By using the value of
The relation between
Explanation of Solution
The value of
Given:
The volume of
An expression used for the neutralization is as follows,
Here,
Substitute
Rearrange for
Therefore, the volume of the
The calculation of moles is done by using the expression,
The ICE table (2) for the reaction between
From the ICE table (2),
A number of moles of ammonia left after reaction is
A number of moles of ammonium ion produced after the reaction is
The total volume after the reaction is calculated as,
Therefore, total volume after the reaction is
Concentration calculations are done by using the expression,
Calculate the concentration of ammonium ion after the reaction.
Substitute,
The concentration of Ammonium ion after the reaction is
The ammonium ion produced will undergo hydrolysis in presence of water and the reaction equilibrium is written as,
The hydrolysis equilibrium is represented in ICE table (3).
From the ICE table (3),
The concentration of ammonium ion left after the reaction is
The approximation
So, Concentration of ammonium ion left after the reaction is
The concentration of ammonia produced after the reaction is
The concentration of
Calculate the concentration of
Rearrange it for
Substitute
Therefore,
The expression of
Substitute,
Rearrange for
Therefore the value of
Calculate the value of
Substitute,
Therefore, the value of
(c)
Interpretation:
The value of
Concept introduction:
Titration is a quantitative method to determine the quantity of an acid or base in a solution. This method is used to determine the concentration an acid in the solution by titrating it against a base.
For strong acid-weak base titration, the
Calculation of
(1) The
(2) The
As the addition of
At the midpoint of the titration, the concentration of acid and its conjugate base is equal. Therefore
Substitute,
Therefore,
(3) The
At equivalence point, all the base will be neutralized, and there will be only
By using the value of
The relation between
Explanation of Solution
The
Given:
The value of
The value of
The
The relation between
Rearrange it for
Substitute
Therefore,
Substitute,
Therefore,
At the midpoint of the titration, the concentration of acid and its conjugate base will be equal.
Substitute
Therefore, the value of
(d)
Interpretation:
The value of
Concept introduction:
Titration is a quantitative method to determine the quantity of an acid or base in a solution. This method is used to determine the concentration an acid in the solution by titrating it against a base.
For strong acid-weak base titration, the
Calculation of
(1) The
(2) The
As the addition of
At the midpoint of the titration, the concentration of acid and its conjugate base is equal. Therefore
Substitute,
Therefore,
(3) The
At equivalence point, all the base will be neutralized, and there will be only
By using the value of
The relation between
Explanation of Solution
Methyl red and bromcresol green are the best indicators for the titration between ammonia and hydrochloric acid. As it changes its color in the
(e)
Interpretation:
The value of
Concept introduction:
Titration is a quantitative method to determine the quantity of an acid or base in a solution. This method is used to determine the concentration an acid in the solution by titrating it against a base.
For strong acid-weak base titration, the
Calculation of
(1) The
(2) The
As the addition of
At the midpoint of the titration, the concentration of acid and its conjugate base is equal. Therefore
Substitute,
Therefore,
(3) The
At equivalence point, all the base will be neutralized, and there will be only
By using the value of
The relation between
The value of
Graph between
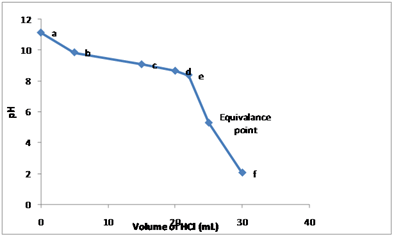
Explanation of Solution
At various points, before the equivalence point, the value of
Given:
When
After titration volume will be equal for both acid and base. The total volume will be
The calculation is done by using equation (1),
Substitute
Therefore the value of
When
After titration volume will be equal for both acid and base. The total volume will be
The calculation is done by using equation (1),
Substitute
Therefore the value of
When
After titration volume will be equal for both acid and base. The total volume will be
The calculation is done by using equation (1),
Substitute
Therefore the value of
When
After titration volume will be equal for both acid and base. The total volume will be
The calculation is done by using equation (1),
Substitute
Therefore the value of
When
After titration volume will be equal for both acid and base. The total volume will be
After the equivalence point, the excess concentration of
The concentration of hydrogen ion after the equivalence point is calculated.
Therefore, the concentration of hydrogen ions after the reaction is
Calculate the
Therefore the value of
The titration curve is drawn by using the
The table gives the value of
Graph between
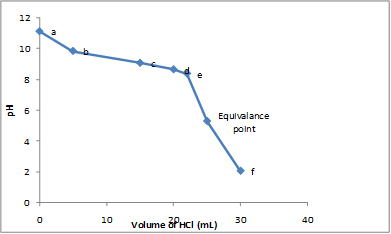
Want to see more full solutions like this?
Chapter 17 Solutions
Chemistry & Chemical Reactivity
- When 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence point is reached when 35.00 mL base has been added. After 20.00 mL NaOH solution has been added, the titration mixture has a pH of 5.75. Calculate the ionization constant of the acid.arrow_forwardConsider the titration of HF (K a=6.7104) with NaOH. What is the pH when a third of the acid has been neutralized?arrow_forwardA 25.0-mL sample of hydroxylamine is titrated to the equivalence point with 35.8 mL of 0.150 M HCl. a What was the concentration of the original hydroxylamine solution? b What is the pH at the equivalence point? c Which indicators, bromphenol blue, methyl red, or phenolphthalein, should be used to detect the end point of the titration? Why?arrow_forward
- Ka for formic acid is 1.7 104 at 25C. A buffer is made by mixing 529 mL of 0.465 M formic acid, HCHO2, and 494 mL of 0.524 M sodium formate, NaCHO2. Calculate the pH of this solution at 25C after 110 mL of 0.152 M HCl has been added to this buffer.arrow_forwardWhat is the pH of a buffer that is 0.175 M in a weak acid and 0.200 M in the acids conjugate base? The acids ionization constant is 5.7 104.arrow_forwardYou are given the following acidbase titration data, where each point on the graph represents the pH after adding a given volume of titrant (the substance being added during the titration). a What substance is being titrated, a strong acid, strong base, weak acid, or weak base? b What is the pH at the equivalence point of the tiration? c What indicator might you use to perform this titration? Explain.arrow_forward
- What is the pH of the solution obtained by titrating 1.30 g of sodium hydrogen sulfate, NaHSO4, dissolved in 50.0 mL of water with 0.175 M sodium hydroxide until the equivalence point is reached? Assume that any volume change due to adding the sodium hydrogen sulfate or to mixing the solutions is negligible.arrow_forwardA 0.400-g sample of propionic acid was dissolved in water to give 50.0 mL of solution. This solution was titrated with 0.150 M NaOH. What was the pH of the solution when the equivalence point was reached?arrow_forwardYou want to make a buffer with a pH of 10.00 from NH4+/NH3. (a) What must the [ NH4+ ]/[ NH3 ]ratio be? (b) How many moles of NH4Cl must be added to 465 mL of an aqueous solution of 1.24 M NH3 to give this pH? (c) How many milliliters of 0.236 M NH3 must be added to 2.08 g of NH4Cl to give this pH? (d) What volume of 0.499 M NH3 must be added to 395 mL, of 0.109 M NH4Cl to give this pH?arrow_forward
- Marble is almost pure CaCO3. Acid rain has a devastating effect on marble statuary left outdoors. Assume that the reaction which occurs is CoCO3(s)+ H+(aq)Ca2+(aq)+HCO3(aq) Neglecting all other competing equilibria and using Tables 15.1 and 13.2, calculate (a) K for the reaction. (b) the molar solubility of CaCO3 in pure water. (c) the molar solubility of CaCO3 in acid rainwater with a pH of 4.00.arrow_forwardA sodium hydrogen carbonate-sodium carbonate buffer is to be prepared with a pH of 9.40. (a) What must the [ HCO3 ]/[ CO32 ]ratio be? (b) How many moles of sodium hydrogen carbonate must be added to a liter of 0.225 M Na2CO3 to give this pH? (c) How many grams of sodium carbonate must be added to 475 mL of 0.336 M NaHCO3 to give this pH? (Assume no volume change.) (d) What volume of 0.200 M NaHCO3 must be added to 735 mL of a 0.139 M solution of Na2CO3 to give this pH? (Assume that volumes are additive.)arrow_forwardA solution is prepared by dissolving 0.350 g of benzoic acid, HC7H5O2, in water to make 100.0 mL of solution. A 30.00-mL sample of the solution is titrated with 0.272 M KOH. Calculate the pH of the solution (a) before titration. (b) halfway to the equivalence point. (c) at the equivalence point.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





