
Concept explainers
(a)
Interpretation: Major organic product in below reaction should be indicated.
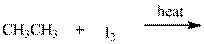
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation:Major organic product in below reactionshould be indicated.
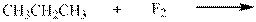
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation: Major organic product in the below reaction should be indicated.
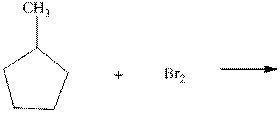
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to the donation of
(d)
Interpretation:Major organic product in the below reaction should be indicated.
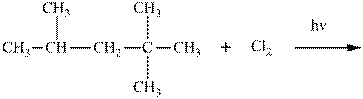
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to the donation of
(e)
Interpretation: Major organic product in below reaction should be indicated.
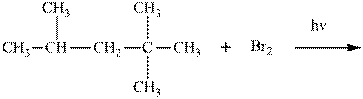
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to the donation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- . Give the major product or products of the following reactions. If there is more than one product indicate the major product. Give the IUPAC names of all reactants products.arrow_forwardWrite the structure of the products of the following reactions.arrow_forwardComplete the following organic reactions and draw structural diagrams to represent the products. a) CH3CH(CH3)CH2COOCH3 + H2O → b) methyl propene + HCl(aq) →arrow_forward
- Name the type of organic compound formed by each ofthe following reactions.a. elimination from an alcoholb. addition of hydrogen chloride to an alkenec. addition of water to an alkened. substitution of a hydroxyl group for a halogen atomarrow_forwardDescribe the intermediate that is thought to form in the addition of a hydrogen halide to an alkene, using cyclohexene as the alkene.arrow_forwardOrganic Chemistry (major/minor products + type of reaction)arrow_forward
- The -COOH functional group characterizes which family of organic compounds? alcohols aldehydes amines carboxylic acidsarrow_forwardWrite the organic products for the following reactionsarrow_forwardNitric acid, Na2Cr2O7 in H2SO4 and KMnO4 will oxidize primary alcohols to ___________. esters aldehydes ketones glycols carboxylic acidsarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


