
Interpretation:Potential-energy/reaction-coordinate diagrams for the two propagation steps of the radical bromination of benzene should be sketched.
Concept introduction: Analogous to hydrocarbons the benzene can also undergo initiation to generate bromine radicals; propagation of radicals formed and finally termination. This sequence can be outlined as follows:
Step1: Initiation via homolytic cleavage of
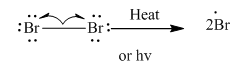
Step2: Propagation: In first of the propagation steps, bromine radical from step 1 abstracts hydrogen radical from
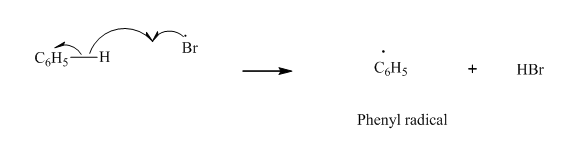
In subsequent propagation step,phenyl radical abstracts
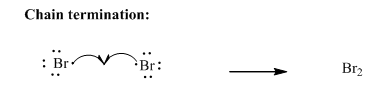
Step3: Termination: Bromine radicals generated in propagation steps get quenched upon combination with one another illustrated as follows:
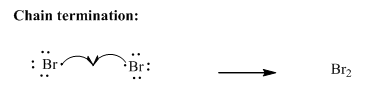
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Explain the Summary of Factors That Determine Whether the SN1 or SN2 Mechanism Occurs ?arrow_forwardDescribe the three stages (initiation, propagation and termination) of radical reactions. Provide also a sample diagram (illustration) for these reactions.arrow_forward1,1-dichloroethane is the product of free radical substitution reaction of ethane. Show the overall mechanism including initiation, propagation and termination steps.arrow_forward
- A possible alternative mechanism to that shown in Problem 47 for the monochlorination of methane involves the following propagation steps: How do you know that the reaction does not take place by this mechanism?arrow_forwardUsing fishhook arrows to propose an initiation step for the reaction. Light (hν) induces homolytic cleavage of a weak bond. The Sn-H bond is rather weak and is therefore highly susceptible to homolytic cleavage.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and zero order in basearrow_forward
- Define mechanism of haloform reaction ?arrow_forward-Draw the transition state showing the allowed homo and lumo interaction of the mechanism -Draw the transition state showing the forbidden Homo/Lumo interaction for the mechanismarrow_forwardplease help me with the question below: Consider Table 4 and suggest why BrCH2CH2Br (35.8 vs. 5.7) has a higher SN2 reaction rate than FCH2CH2Br.arrow_forward
- Consider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and first order in base.arrow_forwardIn an SN2 mechanism, how does it work, what are all of the steps, transition state and all final products?arrow_forwardWhat is the mechanism of the stille reaction?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
