
Concept explainers
(a)
Interpretation: The initiation, propagation and termination step of treatment of methane with hydrogen peroxide should be written and
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive then system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(b)
Interpretation: The value of
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive, the system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(c)
Interpretation: The value of
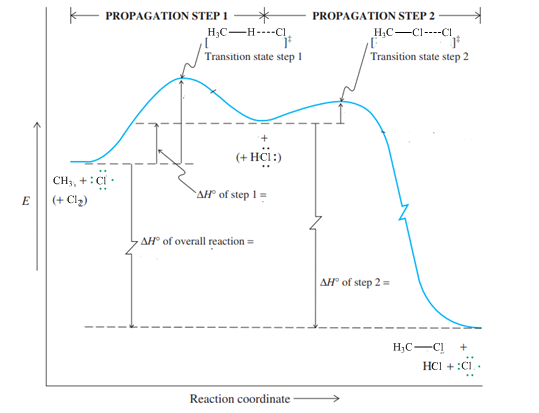
Concept introduction:Hammond postulate states that exothermic reactions are characterized by early transition states that resemble the structure of substrate more than the product. Slow or endothermic processes are characterized by late transition states that resemble the almost formed bonds as in the products.
(d)
Interpretation: The reaction that is faster among reaction of methane with chlorine or peroxide should be identified.
Concept introduction:Hammond postulate states that exothermic reactions are characterized by early transition states that resemble the structure of substrate more than the product. Slow or endothermic processes are characterized by late transition states that resemble the almost formed bonds as in the products.The energy profile diagram relates the barrier required to attain a transition state as illustrated below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Following is a balanced equation for the allylic bromination of propene. CH2==CHCH3 + Br2 h CH2==CHCH2Br + HBr (a) Calculate the heat of reaction, H 0, for this conversion. (b) Propose a pair of chain propagation steps and show that they add up to the observed stoichiometry. (c) Calculate the H 0 for each chain propagation step and show that they add up to the observed H 0 for the overall reaction.arrow_forwardDefine the Mechanism of the Radical Addition of HBr to an Alkene ?arrow_forward2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forward
- Consider the compound 3(S),5,5-trimethyl cyclohexane. Upon reduction with H2 on a 1% Pt/C catalyst, draw the equation for this reaction, find out the absolute configuration of the product and explain why the absolute configuration is completely inverted in this reactionarrow_forward1. a) Write an equation (showing all reaction conditions) for the preparation of ethylene (C2H4) from the dehydration of ethanol. 2.) The reaction above (1.a) can also produce ether under certain conditions. Write an equation to show this reaction as well.arrow_forwardwhich would be expected to convert 1 mole of 4-methyl-1-pentyne into 2-methylpentane? Na, NH3(l) 2 moles H2, Pt 1 mole H2, Pt 2 moles of HCl H2, Lindlar’s catalystarrow_forward
- Alkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C4H9)3SnH, in the presence of light (hv). Propose a radical chain mechanism by which the reaction might occur. The initiation step is the light-induced homolytic cleavage of the Sn-H bond to yield a tributyltin radical.arrow_forwardIn cells, vitamin C exists largely as its conjugate base X. X is an antioxidant because radicals formed in oxidation processes abstract the labeled H atom, forming a new radical that halts oxidation. Draw the structure of the radical formed by H abstraction, and explain why this H atom is most easily removed.arrow_forwardHow does the first step in the reaction of propene with Br2 differ from the first step in the reaction of propene with HBr?arrow_forward
- Predict the major products formed when 2- methyl-1-butene reacts with: Cl2. Show the reaction mechanism the given alkene reactionsarrow_forwardAlkoxy radicals (R¬O # ) are generally more stable than alkyl (R # ) radicals. Write an equation showing an alkyl free radical (from burning gasoline) abstracting a hydrogen atom from tert-butyl alcohol, (CH 3)3COH. Explain why tert-butyl alcohol works as an antiknock additive for gasoline.arrow_forwardWrite the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethanearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

