
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 36P
Interpretation Introduction
Interpretation: The mechanism of chlorination of methane with
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
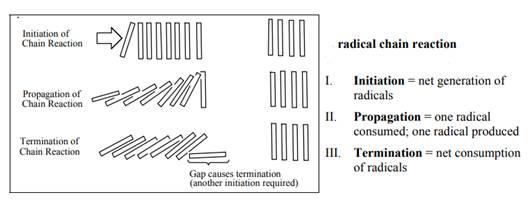
The fundamental radical chain mechanism is summarized in the illustration below:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For parts b and c could you please explain why there is a cis isomer formed and how to know when there will be one. For part e do you have to rearrange the compound to achieve the antiperiplanar orientation?
Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product. (b) 2-chlorobutane (c) 3-bromopentane
(e) trans-1-bromo-2-methylcyclohexane.
Drawing on what you know about the stereochemistry of alkene addition reactions, a. write the mechanism for the reaction of 2-butyne with one equivalent of Br2.
A. predict the configuration of the product of the reaction.
A chemist wanted to determine experimentally the relative ease of removing a hydrogen atom from a tertiary, a secondary, and a primary carbon by a chlorine radical. He allowed 2-methylbutane to undergo chlorination at 300 °C and obtained as products 36% 1-chloro-2-methylbutane, 18% 2-chloro-2-methylbutane, 28% 2-chloro-3-methylbutane, and 18% 1-chloro-3-methylbutane. What values did he obtain for the relative ease of removing a hydrogen atom from tertiary, secondary, and primary hydrogen carbons by a chlorine radical under the conditions of his experiment?
Chapter 3 Solutions
Organic Chemistry: Structure and Function
Ch. 3.1 - Prob. 3.2TIYCh. 3.1 - Prob. 3.3ECh. 3.4 - Prob. 3.5TIYCh. 3.5 - Prob. 3.6ECh. 3.7 - Prob. 3.7ECh. 3.7 - Prob. 3.9TIYCh. 3.9 - Prob. 3.11TIYCh. 3.11 - Prob. 3.12ECh. 3 - Prob. 15PCh. 3 - Prob. 16P
Ch. 3 - Prob. 17PCh. 3 - Prob. 18PCh. 3 - Prob. 19PCh. 3 - Prob. 20PCh. 3 - Prob. 21PCh. 3 - Prob. 22PCh. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Prob. 29PCh. 3 - Prob. 30PCh. 3 - Prob. 31PCh. 3 - Prob. 32PCh. 3 - Prob. 33PCh. 3 - Prob. 34PCh. 3 - Prob. 35PCh. 3 - Prob. 36PCh. 3 - Prob. 37PCh. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Prob. 41PCh. 3 - Prob. 42PCh. 3 - Prob. 43PCh. 3 - Prob. 44PCh. 3 - Prob. 45PCh. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48PCh. 3 - Prob. 49PCh. 3 - Prob. 50PCh. 3 - Prob. 51P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a mechanism for the free-radical chlorination of ethane,CH3¬CH3 + Cl2 ¡hv CH3¬CH2Cl + HClarrow_forwardFollowing is a balanced equation for bromination of toluene. (a) Using the values for bond dissociation enthalpies given in Appendix 3, calculate H0 for this reaction. (b) Propose a pair of chain propagation steps and show that they add up to the observed reaction. (c) Calculate H0 for each chain propagation step. (d) Which propagation step is rate-determining?arrow_forward(A) Show the steps in the mechanism for the conversion of butane to 2-bromobutane using Br2 in the presence of UV light. Be sure to identify initiation, propagation, and termination steps. (B) Starting with toluene (methylbenzene) and any other inorganic or organic reagents or catalysts, show a series of reactions (with reagents) for the synthesis of benzyl ethanoate (benzyl acetate).arrow_forward
- show the mechanism for the alkylation of benzene by 2-butene + HF.arrow_forwarduse the following reagents and show the mechanism 1. AlBr3 2. CH3NO2arrow_forwardThis is a practice problem that I am trying to figure out. Could you please help me to understand and check my work? 1)Draw the structures for all monochlorination products formed by the radical chlorination of 2,2,4- trimethylpentane. 2) Using the relative reactivity below of 5:4:1 for chlorination of 1o, 2o, 3o C-H positions, predict the product ratios for the monochlorination compounds identified. 3) For each product indicate if the molecule is chiral or achiral, how many magnetically distinct hydrogens exist in each product and for the major product predicted, indicate the expected multiplicity for each hydrogen in the NMR.arrow_forward
- Phineas and Ferbs, two brothers who enjoy vacations, doing fun things every summer. This summer the brothers and their friends carry out an organic synthesis with an unknown compound (L1) that contains 52% Carbon, 6% Hydrogen and 42% bromine, this compound (L1) is treated with magnesium in ether to obtain L2 , which reacts violently with D2O for 1-methyl cyclohexene with a deuterium atom in the methyl group (L3). The L2 reaction is treated with acetone followed by hydrolysis to give L4. Heating L4 with concentrated sulfuric acid gives L5, which decolors the bromine, obtaining L6. L5 undergoes hydrogenation with excess hydrogen and platinum as a catalyst giving rise to isobutyl cyclohexane. Determine the structures of compounds L1 through L6.arrow_forward1.- Why are carbon nanotubes and graphene currently considered as reinforcing additives for polymers? What effect do they have on the polymer? What is the mechanism by which they can enhance the Young’s modulus and tensile strength of a polymer? 2.- Kapton is the trade name of a polyimide a) How would you synthesize this polymer? b) During the first stage of the reaction, poly(amic acid) is formed. If you need to follow this stage of the polymerization, what spectroscopic techniques would you use and what groups should you focus on? c) How would you form Kapton films, which can be used as support for solar sails? d) What are the disadvantages of Kapton that have limited their use? Why does this polymer present this weakness?arrow_forwardDrawing on what you know about the stereochemistry of alkene addition reactions, a. write the mechanism for the reaction of 2-butyne with one equivalent of Br2. b. predict the configuration of the product of the reaction.arrow_forward
- I’m currently trying to write a lab report for the synthesis of dimolybdenum tetraacetate [Mo2(O2CCH3)4] from the reaction of molybdenum hexacarbonyl, Mo(CO)6 in glacial acetic acid and acetic anhydride under a nitrogen atmosphere, involving the difficult formation of a quadruple bond and requires high heat and long reaction time (approximately 20 hours). But these are the question I’m stumped on: 1. Why need the reaction be done under nitrogen? We also added dichlorobenzene and hexanes during the reaction. 2. Explain the purpose of dichlorobenzene and hexanes. 3. Why does the reaction take 20 hrs?arrow_forwardNaturally occurring compounds called terpenoids, which we'll discuss in Section 27-5, are biosynthesized by a pathway that involves loss of CO2 from 3-phosphomevalonate 5-diphosphate to yield isopentenyl diphosphate. Use curved arrows to show the mechanism of this reaction.arrow_forwardTributyltin hydride (Bu3SnH) is used synthetically to reduce alkyl halides, replacing a halogen atom with hydrogen. Free-radical initiators promote this reaction, and free-radical inhibitors are known to slow or stop it. Your job is todevelop a mechanism,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY