
Concept explainers
(a)
Interpretation: Various products formed from the pyrolysis of butane with regard to only
Concept introduction: Long-chain petroleum hydrocarbons can be broken down unless highly elevated temperatures are used. At extremely elevated temperature the
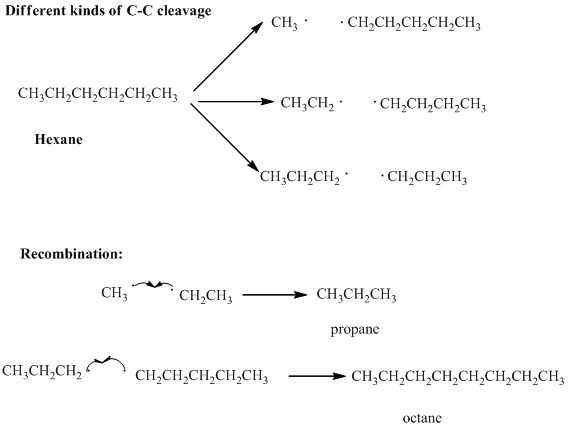
Thus the products have new covalent bonds formed as results of electrons contributed from different atoms.
(b)
Interpretation: Various products formed from the pyrolysis of
Concept introduction: : Long-chain petroleum hydrocarbons can be broken down unless highly elevated temperatures are used. At extremely elevated temperature the
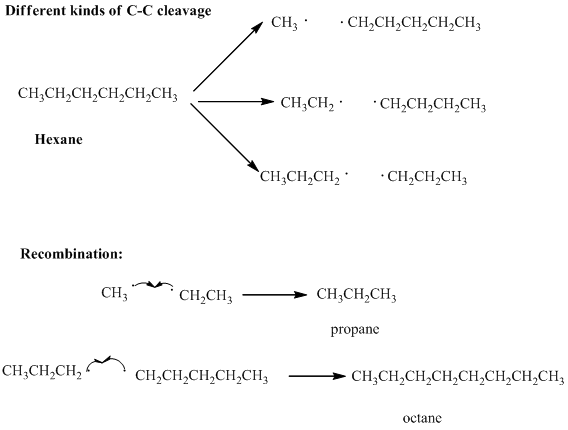
Thus the products have new covalent bonds formed as results of electrons contributed from different atoms.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Consider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Order of reactivity of haloalkanes is methyl . 1° > 2° > 3°.arrow_forwardDraw the mechanism of the hydroboration reaction for 1-octene. Why are carbocation rearrangements not observed in this reaction?arrow_forwardThe electrophilic addition reaction shown below involves a carbocation rearrangement. For the mechanism step below, draw curved arrows to show electron reorganization. Use the arrow tool to specify the origin and the destination of the reorganizing electrons. Consult the arrow-pushing instructions for the convention on regiospecific electrophilic attack on a double bond. I understand how it's supposed to be laid out. I'm just unsure of where the first arrow comes from as there are a lot of positions. I have attached examples on what positions I mean. Thank you.arrow_forward
- idk how to solve part b of this problem.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Order of reactivity of haloalkanes is 3°> 2°> 1°.arrow_forwardPlease help me with the organic chemistry problem below: Consider the reaction below: (Check the attached image) (it is between Furan and maleic anhydride, a DIels-Alder reaction) a) Will this reaction for an endo product (with a melting point of 80-81 degrees) or the exo product (with a melitng point of 114 degrees)? b) Carefully explain why the product must have been formed the way it did (exo or endo). c) Provide a mechanism for this reaction.arrow_forward
- please help me with the question below: Consider Table 4 and suggest why BrCH2CH2Br (35.8 vs. 5.7) has a higher SN2 reaction rate than FCH2CH2Br.arrow_forwardWhat type of mechanism is needed for this reaction?arrow_forwardAlkylbenzenes such as toluene (methylbenzene) react with NBS to give products in which bromine substitution has occurred at the position next to the aromatic ring (the benzylic position). Explain, based on the bond dissociation energies in Table 6-3 on page 170.arrow_forward
- Below is the equation for a nucleophilic substitution reaction and some experimental data. CH3CH2Br + CH3COO- ⇌ CH3CH2CO2CH3 + Br- ΔH=-75 kJ/mol Rate = k [CH3CH2Br][CH3COO-] Which reaction energy profile would be the best representative of the data provided?arrow_forwardhelp me please. all the reactions shall be covered in Chapter 12-15 from Organic Chemistry, 6th Edition by Marc Loudon and Jim Parise. please make sure your answer fits the question; thank you! I posted the same question before and someone gave same answers to all of the questions :(arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and first order in nucleophilearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

