
(a)
Interpretation:Product ratios should be calculated for the indicated reaction.
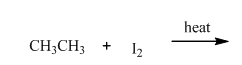
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation:Product ratios should be calculated for the indicated reaction.
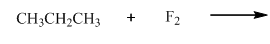
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation:Product ratios should be calculated for the indicated reaction.
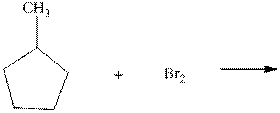
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(d)
Interpretation:Product ratios should be calculated for the indicated reaction.
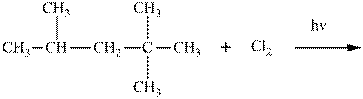
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(e)
Interpretation:Product ratios should be calculated for the indicated reaction.
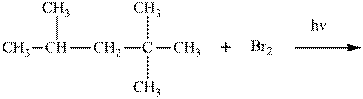
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- The following complexation reaction was carried out by using 2.5 g of cobalt(II)nitrate hexahydrate with 10.0 g of ammonium carbonate and excess of ammonia and hydrogen peroxide. Co(NO3)2.6H2O + 4NH3(aq) + (NH4)2CO3 + ½ H2O2 → [Co(NH3)4CO3]NO3 + NH4NO3 + 6H2O + NH4OH Which is the limiting reagent in the above reaction? Mention how you will identify it. 2. Write the oxidation and reduction half equations of this reaction. 3. How did Werner first explain bonding in complexes? (Refer website)arrow_forwardGive the summary of reaction and rationalization of each samples.arrow_forwardPredict the products of the chemical reaction (select all that apply and pay attention to coefficients).Na2O(s) + H2O(l) →A. Na+B. NaC. 2 OH–D. OH–E. 2 Na+F. 2 NaG. H3O+H. H2O2arrow_forward
- Give equations and explanations for: Randles Sevcik analysis and Brown Anson analysisarrow_forwardRefering to the image attached (Winkler method), what is the function of sodium thiosulphate and why do we need to add in the solution until it turns into pale yellow colour?arrow_forwardWhen butanoic acid (7.0 mL) is dissolved in methanol (20 mL) and heated with a catalytic amount of concentrated sulfuric acid, methyl butanoate (6.5 mL) is isolated. Calculate the per cent yield for the formation of this product. (Use a mole for the process)arrow_forward
- Hello, I do not understand this quiestion or how to do this problem. Can you help me so I can follow along. 7. 5.00 mL of stock solution is diluted to 25.00 mL, peoducing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL resulting in solutin BETA. 10.00 mL of solutionBETA is then diluted 25.00 mL producing solution Gamma. a. Determine the dilution factor in produciing solution Alpha from the stock solution. b. Determine the dilution factor in producing solution Beta from the solution Alpha c. Determie the dilution factor in producing solution Gamma from the stock solution Beta. d. Determine the overall serial dilution factor in producing solution Gamma form the stock solution.arrow_forwardDescribe how to prepare 1L of 150.0ppm cu2+ using cu metalarrow_forwardWhat is the balanced equation for the combustion of C8H7NO2SBrCl in aC,H,N,S elemental analyzer?arrow_forward
- Mass of KxFe(C2O4)y · zH2O : 5.60 g Mass of sample : 0.155 g Mass of FeCl3 used in preparation : 1.60 g Molarity of standard NaOH used : 0.100 V1, volume of standard NaOH required for first equivalence point : 9.000 mL V2, volume of standard NaOH required for second equivalence point : 15.90 mL Calculate the mass of iron in the sample :arrow_forwardCalculate the E0 for the reaction between S2- and Fe3+ that yields SO42- and Fe2+.This was all I was given. I do not know where to start, what I need, or what I need to calculate first. There are no other instructions or parts to this question. I'm at a loss, where do I begin?arrow_forwardKnowing that the concentration of CO2 dissolved in the blood is approximately 1.2 mmol L-1 and that the concentration of bicarbonate (HCO3 - ) is approximately 24 mmol L - 1 , what is the pH of the blood plasma? Write the reactions of the equilibria involved. Data Ka1 of H2CO3 = 4.3x10-7arrow_forward
