
Concept explainers
(a)
Interpretation: Overall equation and the propagation steps of the mechanism for the chlorination of chloromethane to furnish dichloromethane should be written.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
The fundamental radical chain mechanism is summarized in the illustration as follows:
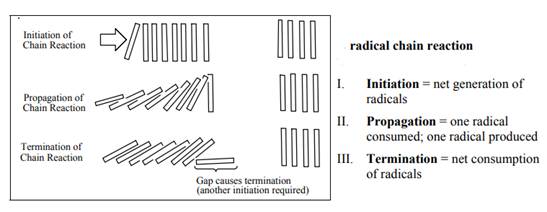
(b)
Interpretation: The formation of small amount of ethane in chlorination of methane should be determined.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorinetransforms
The fundamental radical chain mechanism is summarized in the illustration as follows:
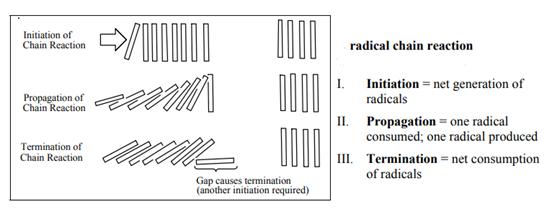
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Define the Mechanism of the Radical Addition of HBr to an Alkene ?arrow_forwardWrite the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethanearrow_forwardCalculate the index of hydrogen deficiency of cyclohexene, C6H10, and account for this deficiency by reference to its structural formula.arrow_forward
- 5. Acetone and 2-propanol are chemically interconvertible. Reduction (by the addition of the equivalent of H2) of acetone yields 2-propanol. Conversely, oxidation (by the removal of the equivalent of H2) of 2-propanol yields acetone. Is the evaporation data for these substances the same or different? If different, can you account for why this may be the case from a comparison of the Lewis structures of each substance and the IMFs present in each substance? Explain.arrow_forwardRead these directions carefully. For the reaction of 3-methyl-1-propene with Cl2 and H2O shown below, fill in the details of the mechanism. Draw the appropriate chemical structures and use an arrow to show how pairs of electrons are moved to make and break bonds during the reaction. Be sure to write all lone pairs of electrons and all formal charges. Finally, in the boxes provided by the arrows, write which kind of mechanistic element is being indicated, such as "make a bond", "add a proton", etc.arrow_forwardWhich is more stable - a phenyl radical, [C6H5]•, or a benzyl radical, [C6H5CH2]• - and why?arrow_forward
- Write a pair of chain propagation steps for the radical bromination of propane to give 1-bromopropane. Then calculate ▲H° for each propagation step and for the overall reaction.arrow_forwardwrite the free radical monosubtitution mechanism for the bromination of ethane to produce bromoethanearrow_forwardWhen exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane. Explain how a mixture is formed from this stoichiometric mixture of reactants, and propose mechanisms for the formation of these compounds from chloromethane.arrow_forward
- Briefly outline the Woodward-Hoffman description and explain how they can be used to predict if a pericyclic reaction will occur thermally or photochemically.arrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forwardWrite down the summary of the selection Rules for Pericyclic reactions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
