
Concept explainers
Interpretation:The greater percentage of anti-conformation in case of
Concept introduction: Various interconvertible forms that result from rotation around the
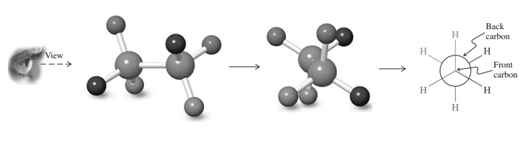
Thus in Newman's projection of simple ethane molecule the “front”
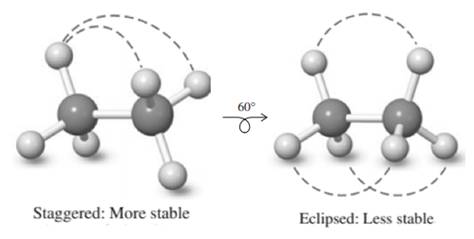
Staggered conformation that has substituents on both carbons farthest apart is regarded most stable due to least torsional strain, whereas the eclipsed has large amount of torsional strain due to steric repulsions. Therefore in potential-energy diagram, peak corresponds to eclipsed while the valley corresponds to stable staggered conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- The cyclohexane derivative shown exists primarily in the more stable of the two available chair conformations. Give the position, axial or equatorial, of each of the three groups shown in the more stable chair conformation. If a group divides its time equally between axial and equatorial positions, indicate this with ax/eq.The table of "Axial Strain Energies for Monosubstituted Cyclohexanes" found in the "Strain Energy Increments" section of the Reference tool is useful for answering this question. For compounds 1 and 2arrow_forwardWrite an example where you can illustrate the application of the Cahn Ingold Prelog rules to assign the stereochemistry around a C = C double bondarrow_forwardIn 1935, J. Bredt, a German chemist, proposed that a bicycloalkene could not have a double bond at a bridgehead carbon unless one of the rings containsat least eight carbons. This is known as Bredt’s rule. Explain why there cannot be a double bond at this position.arrow_forward
- Write TRUE if the BOLD word/phrase makes the statement correct. Otherwise, write the correct WORD/PHRASE that will make the statement true. If there are two bold words/phrases in a number, write your answer for EACH of the bold words/phrases. 1. The anti-staggered conformation of butane, in which the methyl groups have a dihedral angle of less than 180°, has the highest energy. 2. The formation of 2-methylpropene as side product in the synthesis of tert-butyl chloride is a nucleophilic substitution reaction. 3. The generation of tertiary carbocation is the rate-determining step in tert-butyl chloride synthesis.arrow_forwardAspirin, or 2-acetoxybenzoic acid, (C9H8O4) is often synthesised from salicylic acid.(i) Sketch and discuss any changes in the number of possible structural conformations ofaspirin relative to those of salicylic acid. (ii) Re-draw the structure predicted to be the lowest energy conformation of aspirin,indicating any expected stabilising and destabilising interactions. Justify your choice.arrow_forwardup an example (not appearing in this ChemActivity) of a pair of molecules that are a)constitutional isomers, b) conformers. c) configurational stereoisomers.arrow_forward
- DHA is a fatty acid derived from sh oil and an abundant fatty acid in vertebrate brains. Hydrogenation of DHA forms docosanoic acid [CH3(CH2)20CO2H] and ozonolysis forms CH3CH2CHO, CH2(CHO)2 (ve equivalents), and HCOCH2CH2CO2H. What is the structure of DHA if all double bonds have the Z conguration?arrow_forwardN-methylpiperidine has the conformation shown. What does this tell you about the relative steric requirements of a methyl group versus an electron lone pair?arrow_forwardDefine the degree of unsaturation ?arrow_forward
- The NMR spectrum of bromocyclohexane indicates a low field signal (1H) at δ 4.16. To room temperature, this signal is a singlet, but at -75 ° C it separates into two peaks of unequal area (but totaling one proton): δ 3.97 and δ 4.64, in ratio 4.6: 1.0. How do you explain the doubling in two peaks? According to the generalization of the previous problem, what conformation of the molecule predominates (at -75 ° C)? What percentage of the molecules does it correspond to? Solve all parts otherwise down vote and hand written solutionarrow_forwardGive the reagents and observations to distinguish between the following pairs of compounds CH3CH = Ch2 and CH3C≡CH CH3CHO and CH3COCH3 CH3CH2NH2 and CH3CONH2arrow_forwardWhat is the difference between homolysis vs. heterolysis. Show by giving an example.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

