
(a)
Interpretation: The reason behind hydrogen abstraction by chlorine rather than bromine atoms even when equimolar amount of each is present should be explained.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
The fundamental radical chain mechanism is summarized in the illustration below:
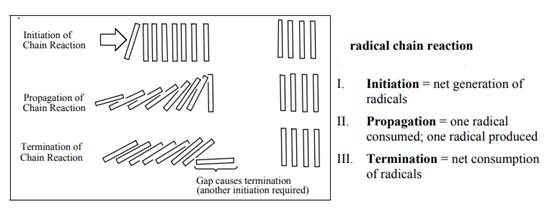
(b)
Interpretation: The bond enthalpies involved in radical halogenation carried out with
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
The formula to calculate
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Write structural formulas for toluene (C6H5CH3) and for benzoic acid (C6H5CO2H) (a) as resonance hybrids of two Kekulé forms and (b) with the Robinson symbol.arrow_forwardWrite a scheme for the reaction of methylamine with hydrogen bromide. Explain how the donor-acceptor bond is formed. Will it differ in properties from other σ-bonds of the nitrogen atom with hydrogen atoms?arrow_forwardInterpret the acidity of alcohols on the basis of ground-state polarization and stability of the alcoholate anion(indicate and give symbols for bond polarization)! Compare the relative acidity of ethanol and 2-fluoroethanol!arrow_forward
- Calculate the index of hydrogen deficiency for 1-hexene, C6H12, and account for this deficiency by reference to its structural formula.arrow_forwardPropose a reasonable mechanism for the reaction, drawing out Lewis structure, using arrows to show electron flow, clearly identifying the bond making/bond breaking steps, and proposing reasonable intermediates for the reaction.arrow_forwardOutline the step-by-step method (initiation, propagation(s), and one termination step) for bromination of 2-bromopropane to produce its major product.arrow_forward
- Cumene -> i. Butyl chloride ii. Dimethyl amine iii. H2, Pt Provide the bond-line structures for the major organic products obtained in each steparrow_forwardWhat is the saturation pressure of benzene at 700°C?arrow_forwardWhich is more stable - a phenyl radical, [C6H5]•, or a benzyl radical, [C6H5CH2]• - and why?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

