
Concept explainers
(a)
Interpretation: Potential-energy versus reaction-coordinate diagrams for the two propagation steps in mechanism for monobromination of pentane should be sketched.
Concept introduction: The mechanism for monobromination comprises of three stages illustrated as follows:
Step1: Initiation via homolytic cleavage of
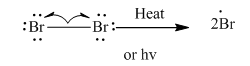
Step2: Propagation: In first of the propagation steps bromine radical from step 1 abstracts hydrogen radical from methane as follows:
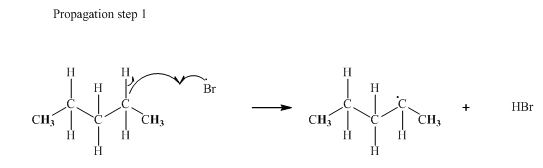
In subsequent propagation step methyl radical abstracts
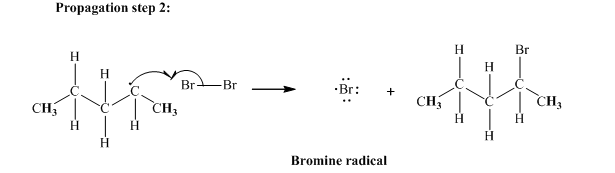
Step3: Termination: Radicals generated in propagation steps get quenched upon combination with one another illustrated as follows:
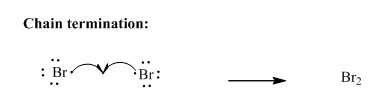
(b)
Interpretation:The locations of the transition states and whether each transition state is early or late should be indicated.
Concept introduction:In general, the exothermic reactions are characterized by an early transition state while the endothermic reaction is characterized by a late transition state.
In the former case, the transfer of
(b)
Interpretation: The similar plot for reaction of pentane with
Concept introduction: In general the exothermic reactions are characterized by an early transition state while the endothermic reaction is characterized by a late transition state.
In the former case, the transfer of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- predict the main product for the addition of 1 equivalent of HX to the following compounds and write the mechanism of the reactionarrow_forward(ii) The elimination reaction between 2-bromobutane and NaOCH2CH3 gives two organic products. Draw a mechanism for the reaction which produces the major organic elimination product and provide a rationale as to why that is the major product.arrow_forwardExplain and detail the reaction mechanism: NaBr + H2O + n-butyl alcohol = ?arrow_forward
- One possible way of determining the identity of an alkene, is to let itundergo an oxidative cleavage reaction in the presence of hot basicpotassium permanganate. You are given two containers said to containdifferent alkenes. Container A is marked as cis / trans‐2‐butene andcontainer B as 2‐methyl‐1‐butene. Explain by referring to the formation ofproducts, how you would verify the identity of the alkenes.arrow_forwardBromine reacts with alkenes in methanol according to the equation (see image 1). When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield) a) Which of the following is the structure more reasonable for this compound? (see image 2) b) Explain your reasoning through a corresponding mechanismarrow_forwardFollowing is a balanced equation for bromination of toluene.(a) Using the values for bond dissociation enthalpies given in Appendix 3,calculate ∆H0for this reaction.(b) Propose a pair of chain propagation steps and show that they add up to theobserved reaction.(c) Calculate ∆H0for each chain propagation step.(d) Which propagation step is rate-determininarrow_forward
- is steric hinderance not an issue here? why when reacting with KOC(CH3)3 does it yield 2-isopropyl-1-pentene and it yields 2,3-dimethyl-2hexene here?arrow_forwardGive 3 examples of a reaction mechanism of E1 that follows Zaitsev's rule.arrow_forwardWhen Br2 is added to buta-1,3-diene at -15 °C, the product mixture contains 60% ofproduct A and 40% of product B. When the same reaction takes place at 60 °C, theproduct ratio is 10% A and 90% B.If you had a solution of pure A, and its temperature were raised to 60 °C, what wouldyou expect to happen? Propose a mechanism to support your prediction.arrow_forward
- Please show the reaction mechanisms of the [3 + 2] cycloadditions of (A) Ozone and (B) OsO4 to cis-2-butene to form their respective 5-membered rings.arrow_forwardThe reaction of 2,2-dimethyl-1-propanol [(CH3)3CCH2OH], also known by the common name neopentyl alcohol, with HBr is very slow and gives 2-bromo-2-methylbutane as the major product.Give a mechanistic explanation for these observations.arrow_forwardPredict the product, draw the mechanism, and plot the reaction coordinate diagram for theE1 reaction.arrow_forward
