
Concept explainers
Spray drying is a process in which a liquid containing dissolved or suspended solids is injected into a chamber through a spray nozzle or centrifugal disk atomizer. The resulting mist is contacted with hot air, which evaporates most or all of the liquid, leaving the dried solids to fall to a conveyor belt at the bottom of the chamber.
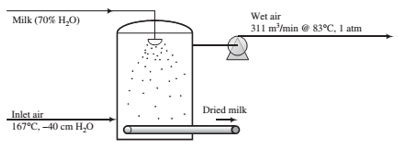
Powdered milk is produced in a spray dryer 6 m in diameter by 6 m high. Air enters at 167°C and −40 cm H2O. The milk fed to the atomizer contains 70% water by mass, all of which evaporates. The outlet gas contains 12 mole% water and leaves the chamber at 83°C and 1 atm (absolute) at a rate of 311 m3/min.
(a) Calculate the production rate of dried milk and the volumetric ?ow rate of the inlet air. Estimate the upward velocity of air (m/s) at the bottom of the dryer.
(b) Engineers often face the challenge of what to do to a process when demand fora product increases (or decreases). Suppose in the present case production must be doubled. (i) Why is it unlikely that the ?ow rates of feed and air can simply be increased to achieve the new production rate? (ii) An obvious option is to buy another dryer like the existing one and operate the two in parallel. Give two advantages and two disadvantages of this option. (m) Still another possibility is to buy a larger dryer to replace the original unit. Give two advantages and two disadvantages of doing so. Estimate the approximate dimensions of the larger unit.
Learn your wayIncludes step-by-step video

Chapter 5 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Introduction to Programming Using Visual Basic (10th Edition)
Introductory Circuit Analysis (13th Edition)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Java: An Introduction to Problem Solving and Programming (7th Edition)
- (5) The emissions from a manufacturing process contain gaseous pollutant that cause odors and fumes. The company plans to incinerate the 5,000 acfm exhaust stream. Estimate the volumetric (standard) flowrate of natural gas fuel required to increase the waste gas stream temperature from the initial 80°F to the required temperature of 2500°F. Energy (enthalpy) is transferred from the fuel to the gas. Neglect any possible heat losses in this process. The average net heating value of natural gas is 850 - 1050 Btu/std-ft. Include a diagram of the scenario.arrow_forwardThe specific heat of compound AB(s) was determined using coffee-cup calorimeter. When 1.750 g of AB(s) was mixed with 15.00 mL deionized distilled water at room temperature, only 0.850 grams of the compound was dissolved. The temperature of the heterogenous mixture was decreased by 1.70K.Prior to this, the calorimeter was calibrated using a 15mL aqueous reaction mixture that initially contains 0.070 moles each of HBr and KOH. The recorded ΔT is +5.75K. Note: H+(aq) + OH -(aq) → H2O(l) ΔH = -55.85 kJ/molAB(s) ⇌ A+(aq) + B-(aq) ΔH = 88.75 kJ/molspecific heat (H2O) = 4.184 J/g°CMM of AB = 65 g/mol What is the specific heat of solid AB compound (in J/g°C)?arrow_forwardConsider the following reaction CS2(g) + 3O2(g) → CO2(g) + 2SO2(g) A mixture containing only CS2(g) and excess O2(g) at a total pressure of 100 kPa is placed in a sealed vessel. After the reaction is completed and the vessel is cooled to the initial temperature, the total pressure in the vessel drops to 80 kPa. What is the mole fraction of CO2(g) in the final mixture?arrow_forward
- A gas mixture at 300K and 1 bar analyzing by volume 20% N2 and 80% CH4 is subjected to liquefaction at the rate of 1500 kg/hr. It is found that only 30% (weight) of the entering gas is liquefied and the concentration of N2 in the liquid is 60% by weight. The unliquefied gas leaves the unit at 273K and 1 bar. Determine (a) the volume of the unliquefied gas, m3/hr (b) the composition of the gas leaving expressed as volume %.arrow_forwardA constant-volume tank initially contains 1 kmol of carbon monoxide CO and 3 kmol of oxygen O2 (no nitrogen) at 25°C and 2 atm. Now the mixture is ignited and the CO burns completely to carbon dioxide CO2. If the final temperature in the tank is 500 K, determine the final pressure in the tank and the amount of heat transfer. Is it realistic to assume that there will be no CO in the tank when chemical equilibrium is reached?arrow_forwardThe synthesis of methanol from carbon monoxide and hydrogen is carried out in a continuous vapor-phase reactor at 5.00 atm absolute. The feed contains cO and H2 in stoichiometric proportion and enters the reactor at 25.0°C and 5.00 atm at a rate of 31.1 m³/h. The product stream emerges from the reactor at 157°C. The rate of heat transfer from the reactor is 21.0 kW. Calculate the fractional conversion (0 to 1) of carbon monoxide achieved and the volumetric flow rate (m3/h) of the product stream. f = Vout ! m3/harrow_forward
- A solid fuel, described by the chemical structure below, is combusted in 10% excess air. If necessary, clearly state any assumptions required to develop your solution. H Н —с — N — с — s — н | || 0 = C S H (a) Determine the mass feed rate (g/min) of the fuel such that 10,000 m³/min of total flue gas is generated at 1500 °C and 1 atm. (b) Determine the effluent SO2 concentration (ppm,) at STP and dry conditions.arrow_forwardConstruct enthalpy cycles; use Hess's law and the following data to calculate the enthalpy of formation of ethane (from carbon and hydrogen gas). Cis) + Ozig) + CO2(g) AHa = -394kJmol H2g) + %02(a) – H20 m AH°. = -286kJmol1 + 3%O2(g) → 2002(9) + 3H20 m AH = -1560kJmol1arrow_forwardThe boiler in an apartment building uses propane (C3H8) to heat water that circulates throughout the building as steam. 1.00 m'/s of propane gas at 25°C and 1 atm is combusted with 10% excess air at 25°C and 1 atm. After all of the propane is combusted, the product gas contains a 10.0:1 molar ratio of CO2 to CO and leaves the boiler at 25°C. Heat from the boiler is transferred to a pure water stream at 50°C and 1.0 bar, which is converted into saturated steam at 20 bar, with no heat lost to the surroundings in the process. What mass of water is heated in this process? Molecule (Formula) (kJ/mol) -393.5 (g) Carbon dioxide (CO2) Carbon -110.52 (g) monoxide (CO) Hydrogen (H2) Nitrogen (N2) Охуgen (O2) Propane (C;Hs) Hot Water (out). Flue Gas Shutoff Valve - Cold Water (in) -119.8(1) -103.8(g) -285.84(1) Gas Supply Line- Cold Water Shutoff Valve Water (H20) -241.83(g) Temperature / Pressure Relief Valve Water Line Overflow Pipe. Tank Insulation - Dip Tube Drain Valve Gas Burner Control-…arrow_forward
- Five moles of monatomic ideal gas enter the abc cycle and during a complete cycle 600 J of heat is removed from the gas. Process ab is under constant pressure and process bc is increasing at constant volume takes place at pressure. The temperatures of points a and b are Ta-= 3°C and Tb= 63°C. Draw the pV diagram of this cycle.arrow_forwardAssume you are interested in exploring the use of ammonium nitrate (NH4NO3) as the active ingredient in an inexpensive home-made cold pack. You decide that a practical cold pack should be able to depress the temperature of 1 kg of human muscle tissue by 5 oC. Now you need to know the amount of NH4NO3 required to achieve this. Ammonium nitrate is a highly water soluble solid. You believe the enthalpy of dissolution for NH4NO3 must be determined. To this end, you add 2.339 g of NH4NO3 to 99.7 mL of water at 23.7 °C in a constant-pressure calorimeter and close the calorimeter. The temperature of the water decreases to a minimum of 21.9 °C. 1a. Assume the density and specific heat of water is 1.00 g/mL and 4.184 J/g °C, respectively. Using the data above, determine the for ammonium nitrate in water. Assume the heat capacity of the calorimeter is negligible.arrow_forwardAssume you are interested in exploring the use of ammonium nitrate (NH4NO3) as the active ingredient in an inexpensive home-made cold pack. You decide that a practical cold pack should be able to depress the temperature of 1 kg of human muscle tissue by 5 oC. Now you need to know the amount of NH4NO3 required to achieve this. Ammonium nitrate is a highly water soluble solid. You believe the enthalpy of dissolution for NH4NO3 must be determined. To this end, you add 2.339 g of NH4NO3 to 99.7 mL of water at 23.7 °C in a constant-pressure calorimeter and close the calorimeter. The temperature of the water decreases to a minimum of 21.9 °C 1) The specific heat of human muscle tissue (smuscle) is 3.47 kJ/kg oC. Given this value, how many grams of ammonium nitrate (NH4NO3) is required to depress the temperature of 1.00 kg of human muscle by 5.00 oC? Please remember, show all work in a legible, highly organized mannerarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





