
5.50.19 In chemical vapor deposition (CVD), a semiconducting or insulating solid material is formed in a reaction between a gaseous species and a species adsorbed on the surface of silicon wafers (disks about 10 cm in diameter and l mm thick). The coated wafers are subjected to further processing to produce the microelectronic chips in computers and most other electronic devices in use today.
In one such process, silicon dioxide (MW = 60.06, SG = 2.67) is formed in the reaction between gaseous dichlorosilane (DCS) and adsorbed nitrous oxide:
A mixture of DCS and N2O ?ows through a “boat reactor"—a horizontal pipe in which 50 to 100 silicon wafers about 12 cm in diameter and 1 mm thick are set upright along the reactor length, with about 20 mm separation between each wafer. A side view of the reactor is shown below:
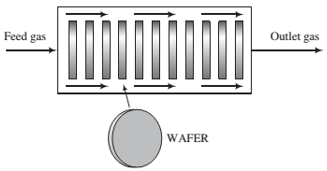
The feed gas enters the reactor at a rate of 3.74 SCMM (standard cubic meters per minute) and contains 22.0 mole% DCS and the balance N2O. In the reactor, the gas ?ows around the wafers, DCS and N2O diffuse into the spaces between the wafers, N2O is adsorbed on the wafer surfaces, and the adsorbed N2O reacts with gaseous DCS. The silicon dioxide formed remains on the surface, and the nitrogen and hydrogen chloride go into the gas phase and eventually leave the reactor with the unconsumed reactants. The temperature and absolute pressure in the reactor are constant at 900°C and 604 millitorr.
(a) The percentage conversion of DCS at a certain axial position (distance along the length of the reactor) is 60%. Calculate the volumetric ?ow rate (m3/min) of gas at this axial position.
(b) The rate of deposition of silicon dioxide per unit area of wafer surface is given by the formula
where
(c) Consider a wafer located at the axial position determined in Part (b). How thick is the silicon dioxide layer on that wafer after two hours of reactor operation, assuming that gas diffusion is rapid enough at the low reactor pressure for the composition of the gas (and hence the component partial pressures) to be uniform over the wafer surface? Express your answer in angstroms, where
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Experiencing MIS
Fundamentals Of Thermodynamics
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Thinking Like an Engineer: An Active Learning Approach (3rd Edition)
- When the high-temperature superconductor yttrium barium copper oxide is heated under flowing H,, the solid remaining at 1000°C is a mixture of Y,O,, BaO, and Cu. The starting material has the formula YBa, Cu,0,-. in which the oxygen stoichiometry varies between 7 and 6.5 (x = 0 to 0.5). 1000°C 1 YBa, Cu,O, -(s) + (3.5 – x)H,(g) FM-666.19–16.00x Y,0, (s) + 2 BaO(s) + 3 Cu(s) +(3.5 – x)H,O(g) YBa, Cu,O35 Thermogravimetric analysis. When 33.792 mg of YBa, Cu, O,, were subjected to this analysis, 31.133 mg of solid remained after heating to 1000°C. Find the value of x in YBa,Cu,O, _r- x = Propagation of error. Suppose that the uncertainty in each mass is ±0.002 mg. Find the uncertainty in the value of x.arrow_forwardThe buildup of oxides of carbon in the atmosphere by the burning of fossil fuels is a driver for human's effect on the world's climate. A chemical solution to this buildup is the removal of these chemicals by reduction to reform småll organic molecules like alkanes and alkenes. Achemist was investigating the removal of carbon dioxide by the following reaction: 2 CO2(g) + 6 H2(8) + C2H5OH(g) + 3 H20(g) Species AfH (kJ mol-1) S(JK-mol1) CO2(g) H2(g) C2H5OH(g) -393.5 213.8 0.00 131.1 -277.4 159.9 H20(g) -241.8 188.8 Using the data from the table above, calculate the enthalpy change, ArH, and entropy change, ArS, for this reaction. Show your working.arrow_forward(a) (c) HO OH 1. BH3 2. H₂O₂, NaOH 3. PCC 4. CH3MgBr 5. H3O+ workup Na₂Cr₂O7 H₂SO4, H₂Oarrow_forward
- At room temperature when HI (aq) is added to potassium bisulfite, KHSO3(s), sulfur dioxide is formed and the reaction vessel becomes cold. HI (aq) + KHSO3(s)→ SO2(g) + H2O(l) + K+(aq) + I-(aq). Fill in the blank: A.) If the temp of the reaction vessel is increased then Ea,rev will... (decrease, stay the same, increase, or need more info). B.) If the temp of the reaction vessel is increased then Kc will... (decrease, stay the same, increase, or need more info).arrow_forwardTungsten is extracted from the mineral scheelite (CaWO4) by roasting it with natrite (Na2CO3) at a temperature of about 700 °C. This converts the CaWO4 to water soluble sodium tungstate (NazWO4). The solid product from the roaster (called "calcine") is transferred to a leacher. In the leacher chilled water is added to dissolve the NazWO4. The chilled water dissolves only the NazWO4 in the solid calcine. The scheelite that enters the roast is mixed with gypsum (CaSO4-2H2O). This stream is 85.2%w CaWO4 and 14.8%w CaSO4-2H2O. (%w means “percent-by-weight.") Four reactions take place during this process. In the roaster: 1: CaWO4 + Na2CO3 - NazWO4 + CaO + CO2 2: CaSO4•2H2O - CaSO4 + 2H2O In the leacher: 3: СаО + H2О —- Са(ОН)2 4: CaSO4 + 2H2O - CaSO4 2H2O The first reaction does not go to completion, all the other reactions do go to completion. For the conditions shown in the process diagram on the next page calculate the conversion of the CaWO4 in the roaster.arrow_forwardThe %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCl solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 torr. Determine the %purity of the sample.arrow_forward
- The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCl solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 torr. a. How many moles of CO2 were collected? b. What is the percent purity of the sample? Round off to the nearest whole numberarrow_forward5R) The net ionic equation for formation of an aqueous solution of Nil2 accompanied by evolution of CO2 gas via mixing solid NICO3 and aqueous hydriodic acid is A) 2NICO3 (s) + HI (aq) - 2H20 (1) + CO2 (g) + 2N12+ (aq) 2H20 (1) + CO2 (g) + Ni2+ (aq) + HI (aq) B) NICO3 (s) + I (aq) O NICO3 (s) + 2H* (aq) H20 (1) + CO2 (g) + Ni2+ (aq) 2H20 (1) + CO2 (g) + Nil2 (aq) D) NICO3 (s) + 2HI (aq) E) NICO3 (s) + 2HI (aq) H20 (1) + CO2 (g) + Ni2+ (aq) + 21- (aq) Answer. Carrow_forwardHematite is an iron ore with the following composition: Fe2O3 [MW=159.70]. To make steel, carbon [At. Wt. = 12.01] in the form of coke is used to reduce Fe2O3 to iron metal [At. Wt. = 55.85] as shown below: 3 C + 2 Fe2O3 → 3 CO2 + 4 Fe How many grams of carbon are needed to produce 2,500 grams of iron? Relative to the problem the processing of 798.5 g of hematite ore produced 508.2 g of iron metal. Determine the percent yield of pure iron for this batch?arrow_forward
- The emission of NO, by fossil fuel combustion can be prevented by injecting gaseous urea into the combustion mixture. The urea reduces NO (which oxidizes in air to form NO2) according to the reaction: 2 CO(NH;)»(3) + 4 NO(3) + O2(8) 4 Na(8) + 2 CO;(g) + 4 H¿O(g) Suppose that the exhaust stream of an automobile has a flow rate of 2.55 L/s at 655 Kand contains a partial pressure of NO of 12.4 torr. What total mass of urea is necessary to react complete- ly with the NO formed during 8.0 hours of driving?arrow_forward1. Railroad tracks made of 1025 steel are to be laid during the time of year when the temperature averages 10°C (50°F). If a joint space of 4.6 mm (0.180 in.) is allowed between the standard 11.9-m- (39-ft-) long rails, (a) what is the hottest possible temperature that can be tolerated without the introduction of thermal stresses? (b) If these tracks were going to be laid in the Australian Outback and see temperatures of 150 °F, what would the stress be?arrow_forwardPure gold crystallizes in a face-centered cubic unit cell with an edge length of 4.08 Å. The alloy called 18-karat gold consists of 75% Au, 8.0% Ag, and 17% Cu by mass. What are the subscripts in the empirical formula of 18-karat gold (Au(x)Ag(y)Cu(z)) to two significant digits?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





