
5.53.20 Chemicals are stored in a laboratory with volume V(m3). As a consequence of poor laboratory practices, a hazardous species, A, enters the room air (from inside the room) at a constant rate 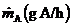 The room is ventilated with clean air ?owing at a constant rate
The room is ventilated with clean air ?owing at a constant rate 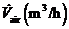 The average concentration of A in the room air builds up until it reaches a steady-state value
The average concentration of A in the room air builds up until it reaches a steady-state value 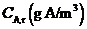
(a) List at least four situations that could lead to A getting into the room air.
(b) Assume that the A is perfectly mixed with the room air and derive the formula
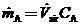
(c) The assumption of perfect mixing is never justi?ed when the enclosed space is a room (as opposed to, say, a stirred reactor). In practice, the concentration of A varies from one point in the room to another: it is relatively high near the point where A enters the room air and relatively low in regions far from that point, including the ventilator outlet duct. If we say that 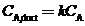 where
where 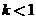 is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
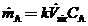
Use this equation and the ideal-gas equation of state to derive the following expression for the average mole fraction of A in the room air:
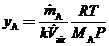
where MA is the molecular weight of A.
(b) The permissible exposure level (PEL) for styrene (M = 104.14) de?ned by the U.S. Occupational Safety and Health Administration is 50 ppm (molar basis).21 An open storage tank in a polymerization laboratory contains styrene. The evaporation rate from this tank is estimated to be 9.0 g/h. Room temperature is 20°C. Assuming that the laboratory air is reasonably well mixed (so that k = 0.5), calculate the minimum ventilation rate (m3/h) required to keep the average styrene concentration at or below the PEL. Then give several reasons why working in the laboratory might still be hazardous if the calculated minimum ventilation rate is used.
(e) Would the hazard level in the situation described in Part (d) increase or decrease if the temperature in the room were to increase? (Increase, decrease, no way to tell.) Explain your answer, citing at least two effects of temperature in your explanation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Concepts Of Programming Languages
C How to Program (8th Edition)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
- perform stoichiometric ca1cu1uions for reactions involving gases as reactants or products.arrow_forward10. Hydrogen peroxide, H.O2, will spontaneously disproportionate into molecular oxygen and water according to the following reaction: 2H.O. - 2H,O. + 0. 5.00 mL sample of 3.0% aqueous hydrogen peroxide is placed in a test tube and reacted according to the above reaction; the oxygen gas is collected over water at 28 °C. a) Assuming the density of the hydrogen peroxide solution is 1.00 g/mlL., what is the mass of hydrogen peroxide in the sample? b) Given the vapor pressure of water at 28 °C is 28.3 torr, what is the volume of oxygen gas isolated from the above reaction if the total pressure is 755.2 torr?arrow_forwardWhen heated strongly, solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas, as represented by the equation above. A 2.0mol sample of CaCO3(s) is placed in a rigid 100.L reaction vessel from which all the air has been evacuated. The vessel is heated to 898°C at which time the pressure of CO2(g) in the vessel is constant at 1.00atm, while some CaCO3(s) remains in the vessel.arrow_forward
- Ideal gas law is used for the working of airbags in vehicles. When airbags are deployed, they are quickly filled with different gases that inflate them. Assume that you are working in a car manufacturing company assigned in the production and installation of the airbags, what will you consider in making the air bags? Explain your reason/s.arrow_forwardConsider the following reaction where Ke = 9.52x10 at 350 K. CHa(9) + CCla(0) =2CH,Cl2(9) A reaction mixture was found to contain 3.07x102 moles of CHa(g), 3.96x10 moles of CCIA(g) and 1.21x102 moles of CH,Cl2(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qo equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forward5. Wine turns into vinegar through the oxidation of ethanol (C2H6O) to acetic acid (C2H4O2) via aerobic bacteria from a range of genera including Acetobacter and Acidiphilium known as acetic acid bacteria (AAB). In a 750 ml wine bottle, there is 730 ml of wine (12% ethanol, by volume) and 20 ml head space. A. Assuming the air trapped in the head space is 21% oxygen (atmospheric O2 at 280 g/m3) and such bacteria are present, how much acetic acid will be formed before the bottle is opened? Half of the energy that is produced is going to biomass build up. Use C8H6N2O for biomass. Assume no sulfites are present in the wine (these are sometimes added to prevent enzymatic activity that drives these reactions). B. If the cork leaks so that the head space is exchanged once per day (20 ml of new air replaces the old every 24 hours), how long will it take for all of the ethanol to be converted to acetic acid, assuming the bacteria survive? Assume no sulfites are present in the wine. Looking…arrow_forward
- 24 mwt (N204) = 92 g/mol 50.0 g of N2O4 is introduced into an evacuated 2.00 L vessel and allowed to come to equilibrium with its decomposition product, N;O4(g) → 2NO:(8). For this reaction Ke = 0.133. Once the system has reached equilibrium, 5.00 g of NO, is injected into the vessel, and the system is allowed to equilibrate once again. Calculate the mass of NO, in the final equilibrium mixture. A. 17.8 g В. 12.4 в С. 14.7 в D. 19.7 g E. 15.5 g O AA O B. B O D. D O EEarrow_forward+ |/ 00 %24 D. Two bulbs are connected by a stopcock. The 7.50 L bulb contains nitric oxide (NO) at a pressure of 0.340 bar, and the 2.50 L bulb contains oxygen (O,) at a pressure of 0.510 bar. 02 ON After the stopcock is opened, the gases mix and react to produce nitrogen dioxide (CON) 2 NO(g) + 0,(g) – 2 NO,(g) Considering that the volume remains unchanged during the experiment, how does the total pressure in the bulbs change if the reaction is allowed to go to completion? The total pressure will remain constant. O There is not enough information to determine how the total pressure will change. O The total pressure will decrease. O The total pressure will increase. MacBook Pro ( The %23 %24 7. 4. 5. 3. R %3D K. H. B C. option command MOSISOarrow_forwardHelp me pleasearrow_forward
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 39.0 cm wide and 46.8 cm high. The maximum safe pressure inside the vessel has been measured to be 1.20 MPa. For a certain reaction the vessel may contain up to 0.560 kg of dinitrogen monoxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits. temperature: | °Carrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 19,0 cm wide and 22.8 cm high. The maximum safe pressure inside the vessel has been measured to be 8.50 MPa. For a certain reaction the vessel may contain up to 0.338 kg of dinitrogen monoxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: c D.Parrow_forwardA chemical manufacturer produces ethylene oxide by burning ethylene gas with air in the presence of catalyst. If the conditions are carefully controlled, a substantial fraction of ethylene remains unconverted and some is completely oxidized to form carbon dioxide and water. Formation of carbon monoxide is negligible. After the gases leaving the absorber is as follows: 9.6%CO2, 3.0%O2 and 6.4% ethylene. Of the ethylene entering the reactor, what percentage is converted to ethylene oxide?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning



