
Concept explainers
(a)
Interpretation:
To sketch the shapes of
Concept introduction:
Metallic Iron is followed by the following reaction.
The flowchart for the process is shown below:
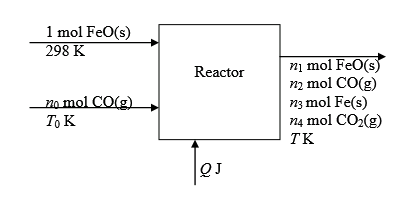
(b)
Interpretation:
To derive the expressions for enthalpies
Concept introduction:
:Metallic Iron is followed by the following reaction.
The flowchart for the process is shown below:
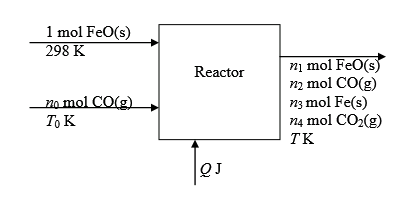
An outlet-enthalpy table is shown below:
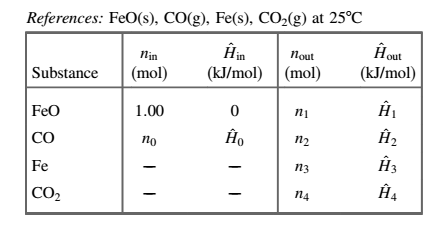
(c)
Interpretation:
To interpret the heat duty
Concept introduction:
Metallic Iron is followed by the following reaction.
The flowchart for the process is shown below:
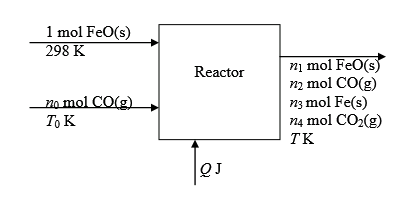
The expressions for enthalpies
(d)
Interpretation:
To prepare the spreadsheet for the given data and shapes of the graphs predicted according to part
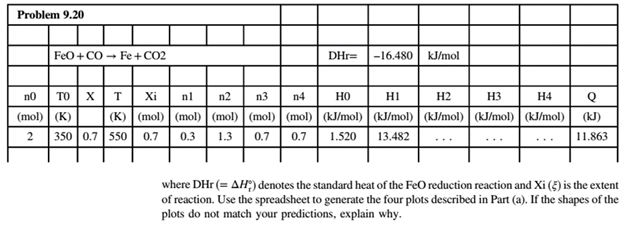
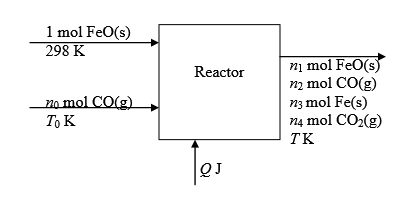
Concept introduction:
:Metallic Iron is followed by the following reaction.
The flowchart for the process is shown below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
- Part F The third variable (call it v) is v=x1-cx2 + x3, where c is a constant you need to determine so that the equation for v is the SHM equation. Determine v. The solution to the equation for vis v = 2B cos(t + b). Express your answer in terms of some, all, or none of the variables 21, 22, 23, and the appropriate constants. v = ΜΕ ΑΣΦ Submit Request Answer ?arrow_forwardFor the reaction bellow: C„H(2n+2) + a(02 + 3.76 N2) → nC02 + (n+ 1)H,0 + 3.76a N2 If the heat of combustion writing as: Qc = 618.49 n+ 187. 83 And the mole fraction of CO, in products is Xco, 1. Type of fuel. 2. Heat of combustion (KJ/mole of fuel) 25 find: 227 3. If n=4, find the mole fractions of Co, H,0 and N2arrow_forward3a. CO₂ can be formed by: BaCO 3 (s) + 2H+¹ (aq) Ba→ܢ +2 (aq) + H2O(liq) + CO2(g) Write the thermodynamic equilibrium constant expression. (4 pts) 3b. K = 3.72 × 10+9 at 1 atm and 298 K. Find AG° in kJ. Show all units and conversion factors. Use significant figures. (4 pts)arrow_forward
- The growth of the bacterium Zymomonas mobilis in ethanol under aerobic conditions is described by the following global reaction:C2H5OH + a O2 + b NH3 → c C H1.704N0.149O0.408 + d CO2 + e H2Oa) Write the equations for the balance by elements and the balance of electrons.b) determine the coefficients a, b, c, and d knowing that RQ = 0.66c) determine YX/S and YX/O2arrow_forwardShow that for a reversible adiabatic process, Note: ● ● ● PVY is constant for an ideal For ideal gases use the ideal gas law: PV = RT Cp You also need to assume that y = Cy is constant. gas. The assumption that y is constant for an ideal gas is equivalent to the assumption that the heat capacities are constant. This is the only way that the ratio and the difference Cp - Cv Cp Cv Cy can both be constant ( = y and Cp - Cv = R). In reality, Cp and Cy increase with temperature, but their ratio y is less sensitive to the temperature than the heat capacities themselves.arrow_forwardYou have a solution containing two metal cations. You want to separate them by slowly and carefully adding a solution of an anion, until one metal cation precipitates as a compound with that anion, leaving the other metal cation still in solution. Your metal cations (M+ and M2+) and your anion M+= Cu^+ M^2+= Cd^2+ Anion= S^2- First Ksp(M+ w/anion)= 2.5 x 10-48 Second Ksp(M2+ w/anion)= 1.0 x 10-28 ----------------------------------------------------------------------------------- Starting concentrations of yourmetal ions in your initial solution: [M+]= 4.00 x 10-5 M [M2+]= 2.50 x 10-5 M Using these values, predict which metal will precipitate first, and at what concentration of anion the precipitation will begin. Clearly state the parameters of your particular problem at the beginning. What is M+, what is M2+, what are your concentrations, what are you solving for?arrow_forward
- The specific heat of compound AB(s) was determined using coffee-cup calorimeter. When 1.750 g of AB(s) was mixed with 15.00 mL deionized distilled water at room temperature, only 0.850 grams of the compound was dissolved. The temperature of the heterogenous mixture was decreased by 1.70K.Prior to this, the calorimeter was calibrated using a 15mL aqueous reaction mixture that initially contains 0.070 moles each of HBr and KOH. The recorded ΔT is +5.75K. Note: H+(aq) + OH -(aq) → H2O(l) ΔH = -55.85 kJ/molAB(s) ⇌ A+(aq) + B-(aq) ΔH = 88.75 kJ/molspecific heat (H2O) = 4.184 J/g°CMM of AB = 65 g/mol What is the specific heat of solid AB compound (in J/g°C)?arrow_forward6A. State whether each of the following is true or false. Explain your reasoning in each case. (a) q must be zero for an isothermal process (b) q=0 for every cyclic process [A cyclic process is one that begins in one state and undergoes several steps which ultimately bring the system back to the same initial state.] (c) DU=0 for every cyclic process. (d) DT=0 for every adiabatic process in a closed system.arrow_forward2 When 50 cm' of hydrochloric acid of concentration 2.0 mol dm is added to 50 cm' of sodium hydroxide solution of concentration 2.0mol dm, the temperature increase is 13.0°C HCl(aq) + NAOH(aq) + NaCl(aq) + H,O(1) The experiment is repeated using 25 cm of the same hydrochloric acid and 50 cm' of the same sodium hydroxide solution. What is the temperature increase? O A 4.9°C I B 6.5°C I C 8.7°C D 13.0°Carrow_forward
- P3D.4 The solubility of an ionic solid such as NaCl can be explored by calculating the standard Gibbs energy change for the process NaCI(s) → Na*(aq) + CI (aq). Consider this process in two steps: (1) NaCI(s) → Na*(g) + CI (g) and then (2) Na*(g) + CI (g) –→ Na*(aq) + CI(aq). Estimate A,G° for the first step given that A,H® = 787kJ mol and the following values of the absolute entropy: S(Na*(g)) = 148JK"mol", S(CI (g) = 154JK'mol", S(NaCI(s)) = 72.1 JK'mol* (all data at 298 K). The value of 4,G° for the second step can be found by using the Born equation to estimate the standard Gibbs energies of solvation. For these estimates, use r(Na*) = 170 pm and r(CI) = 211 pm. Hence find A,G° for the overall process and comment on the value you find.arrow_forward3. Suppose you have a bomb calorimeter and first calibrate it using a known quantity of electrical heat (q=current*voltage*time), thus providing known values for q and AT. Next (using the same device), you measured the combustion of 1 mole of methane, CH4(g) + 2 O2(g) > Co2{g) + 2 H20(1), resulting in a known value of AT, and assuming a perfect gas. Explain with equations and words (be specific), how you would obtain the molar enthalpy of combustion (AcHm ) from this data. Охудen input Firing leads Thermometer Bomb Sample Охудen under pressure Waterarrow_forward7C. The total differential for H(T,P) (where H is enthalpy) is dT + ӘР dH dP Show that dH=C,dT for a process involving an ideal gas even if the pressure is changing. (Note that this requires that you show that (@H/@P),=0 for an ideal gas.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





