
You are checking the performance of a reactor in which acetylene is produced from methane in the reaction
An undesired side reaction is the decomposition of acetylene:
Methane is fed to the reactor at 1500°C at a rate of 10.0 mol CH4/S. Heat is transferred to the reactor at a rate of 975 kW. The product temperature is 1500°C and the fractional conversion of methane is 0.600. A flowchart of the process and an enthalpy table are shown below.
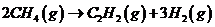
References: C(s), H2(g), at 25°C, 1 atm
| Substance |
|
|
|
|
|
|
10.0 | 41.65 |
|
|
|
|
— | — |
|
|
|
|
— | — |
|
|
| C | — | — |
|
|
(a) Using the heat capacities given below for enthalpy calculations, write and solve material balances and an energy balance to determine the product component flow rates and the yield of acetylene (mol C2H2produced/mol CH4consumed).
For example, the specific enthalpy of methane at 1500°C relative to methane at 25°C is [0.079 kJ/(mol·°C)](1500°C - 25°C) = 116.5 kJ/mol.
(b) The reactor efficiency may be defined as the ratio (actual acetylene yield/acetylene yield with no side reaction). What is the reactor efficiency for this process?
(c) The mean residence time in the reactor [
If the mean residence time increases to infinity, what would you expect to find in the product stream? Explain.
Someone proposes running the process with a much greater feed rate than the one used in Part (a), separating the products from the unconsumed reactants, and recycling the reactants. Why would you expect that process design to increase the reactor efficiency? What else would you need to know to determine whether the new design would be cost-effective?
Trending nowThis is a popular solution!

Chapter 9 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Digital Fundamentals (11th Edition)
Electric Circuits (10th Edition)
Mechanics of Materials
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
- Iodine is not very soluble in water, but it dissolves readily in a solution containing iodide ions by the following reaction: I₂ (aq) + I¯ (aq) → 13(aq) The following graph shows the results of a study of the temperature dependence of AG for this reaction. (The solid line is a best fit to the actual data points.) Notice that the quantity on the y axis is AG /T, not just AG. Additional data relevant to this reaction are also given in the table that follows the graph. AGOT (kJ/K) -0.050 -0.052 -0.054 -0.056 -0.058 -0.060 0.0030 0.0031 0.0032 0.0033 0.0034 0.0035 0.0036 0.0037 1/T (1/K) Substance AG (kJ mol-¹) 1₂ (aq) 16.37 I (aq) 13 (aq) -51.57 -51.4 a Calculate AG for this reaction at 298 K. (DO NOT read this value off the graph. Use the data given to calculate a more accurate value.) AG = kJarrow_forwardConsider the reaction below. The reaction quotient for a specific moment is 3.42-10³. If the equilibrium constant is 1.16. 104, what needs to happen in order for the reaction to reach equilibria? Select all that apply. 2 A+ 4 B5 C There will be a net gain in reactant. There will be a net gain in product. The forward reaction will procede more than the reverse reaction. The reverse reaction will occur more than the forward reaction. The system will shift to the left. The system will shift to the right.arrow_forwardA student dissolves 10.3g of ammonium nitrate NH4NO3 in 300.g of water in a well-insulated open cup. He then observes the temperature of the water fall from 22.0°C to 19.3°C over the course of 3.1 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH4NO3s -> NH+4aq + NO−3aq You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy ΔHrxn per mole of NH4NO3.arrow_forward
- 2. The predominant forms of heavy metals (Zn, Pb, Fe, Cu, Ni, etc.) in aerobic sediments are the carbonates and the oxide or hydroxide forms. An important consideration in modeling the sediment is the stable chemical form of the metal in question. Consider the reaction: AG = 25 kJ/mol Pb(OH)₂ (s) + CO₂(aq) → PbCO₂ (s) + H₂O a. At what partial pressure of CO₂(g) will Pb(OH)2(s) and PbCO3(s) be at equilibrium? b. If the system is at equilibrium with the atmosphere, Pc02-10-3.38 atm, calculate 4G for the reaction. What is the stable form of lead in the sediments?arrow_forwardA stoichiometric mixture of CO(g) and H2(g) was allowed to react in two different 2.0L rigid containers at a constant temperature of 298K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed. Which of the following correctly explains the experimental results represented in the particle diagrams? A.) Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because its reactant molecules had a higher average kinetic energy. B.) Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because it has a lower activation-energy reaction pathway. C.) The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but the addition of a catalyst improved the…arrow_forward1. A reactor with rigid walls (volume 8 I) is filled with 250 ml of oxygen (at 15 atm and 40°C) and 500 ml of CO (at 20 atm and 30°C). Then the conversion of CO to CO₂ occurs. Calculate the partial pressure and the total pressure at the end of the reaction knowing that the temperature reaches 223°C.arrow_forward
- Nitrogen tetroxide breaks down according to the equation: N204 (g) +2NO2 (g) AH = 54KJ / mol %3D Pure N204 (g) was added to a 3.00 L container at 127 ° C under a pressure of 5.00 kPa. After the system reached its equilibrium state, the total pressure in the container was found to have risen to 8.43 kPa. Question 1: Calculate the value of KP at 127 oC for this reaction Question 2: The volume of the container is suddenly compressed to 1.00 L at 127 ° OC, tripling the pressure of all components in the container from equilibrium in (a). In what direction would the equilibrium of the reaction shift? Calculate the new partial pressures of N204 and NO2, as well as the total pressure in the container, once equilibrium is re- establishedarrow_forwardcellus_engine.html?ClassID=2134898241# Manipulate equation 2 to match the NH3 part of the overall equation. What will be the new AH value? 2) N2(g) + 3H2(g) → 2NH3(g) AH = -91.8 kJ !! goal equation: 4NH3(g) + 502(g) 4NO(g) + 6H2O(g) AH° = [ ? ] kJ Enter either a + or - sign AND the magnitude. Do not round. Enterarrow_forwardConsider the reaction below: A + 2B → C You carry out a reaction using .100 moles A and .150 moles of B in a calorimeter with Ccal = 4.12 kJ/°C. Given the data below, what is the enthalpy of reaction in kJ/mol? Temperature (°C) 26 25 24 23 22 -110 0 30 60 Time (s) 90 Check the sign of AH, make sure you scale by moles of reaction.arrow_forward
- What is AS for the combustion of propane? C3H8(g) +502(g) → 3CO2(g) + 4H₂O(g) Substance C3H8(g) O2(g) CO2(g) H₂O(g) S(J/K mol) 269.9 205.0 213.6 188.7arrow_forward< 1 4 7 +/- Given the following reaction: 2C₂H₂(g) +50₂(g) → 4CO₂(g) + 2H₂O(g) AH = -2511.6 kJ What is the energy change when 2.26 g of C₂H₂ react with excess O₂? 2 LO Question 11 of 25 5 8 KJ 3 6 9 O Submit Tap here or pull up for additional resources X C x 100arrow_forwardunity College - CHEM 1020 - Spring20 - NGWENDSON > Activities and Due Dates > Ch 7 HW Najma Jama - く Assignment Score: 42.6% Resources < Question 17 of 23 O Hint Check Answer Kス Consider the combustion reaction for octane (C, H), which is a primary component of gasoline. 2 C3H18 + 25 O,→ 16 CO, + 18 H,O How many moles of CO, are emitted into the atmosphere when 11.6 g C,H3 is burned? CO, emitted: mol CO2 about us privacy policy terms of use contact us help careers 13 59 PACES APR Aa POF 16 DD F12 DII F11 F10 F9 000 F8 F7 F6arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





