
Hydrogen is produced in the steam reforming of propane:
The water-gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen:
The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125°C, and the products emerge at 800°C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing the exhaust gas from a nearby boiler over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m3/mol C3H8, entering the unit at 1400°C and 1 atm and leaving at 900°C. The unit may be considered adiabatic.
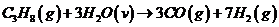
Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040 kJ/(mol·°C).
- Is the reaction process exothermic or endothermic? Explain how you know. Then explain how running the reaction in a reactor-heat exchanger improves the process economy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Fundamentals Of Thermodynamics
DeGarmo's Materials and Processes in Manufacturing
Management Information Systems: Managing the Digital Firm (15th Edition)
- 2. Suppose you performed a similar experiment using an unknown sample of carbonate. The pressure and volume were measured after the temperature stabilized at 21.5 °C. Given the data in the table below, what is the stoichiometric ratio between HCl(aq) and CO2(g)? Metal carbonate + HCl(aq) = Metal chloride + CO2(g) + H2O(1) Initial pressure (atm) 1.0 Initial gas volume (mL) 240.0 Volume of 1.0 M HCl solution added to fully consume the carbonate sample (mL) 22.0 Final pressure (atm) 2.32 Final gas volume (mL) 218.0arrow_forwardYou want to determine the %composition of iron in a soil sample. You dissolved it in LIOH, forming solid FeOH. Then you subjected your sample in the furnace forming FeO. You weighed the FeO and compared it to the original mass of the sample. What type of gravimetry is described in the statement? thermogravimetry electrogravimetry precipitation gravimetry physical gravimetryarrow_forwardThe emission of NO, by fossil fuel combustion can be prevented by injecting gaseous urea into the combustion mixture. The urea reduces NO (which oxidizes in air to form NO2) according to the reaction: 2 CO(NH;)»(3) + 4 NO(3) + O2(8) 4 Na(8) + 2 CO;(g) + 4 H¿O(g) Suppose that the exhaust stream of an automobile has a flow rate of 2.55 L/s at 655 Kand contains a partial pressure of NO of 12.4 torr. What total mass of urea is necessary to react complete- ly with the NO formed during 8.0 hours of driving?arrow_forward
- Coal-fired power plants emit CO2, which is one of the gases that is of concern with regard to global warming. A technique that power plants can adopt to keep most of the CO2 from entering the air is called CO2 capture and storage (CCS). If the incremental cost of the sequestration process is $0.019 per kilowatt-hour, what is the present worth of the extra cost over a 3-year period to a manufacturing plant that uses 100,000 kWh of power per month? The interest rate is 12% per year compounded quarterly.arrow_forwardWrite a balanced chemical equation based on the following description: butanoic acid, C,H,COOH(I) burns in air | What type of reaction is represented by the following equation: 2Na(s) + Cl2(g) → 2NACI(s) A) combination B) decomposition C) double displacement D) single displacementarrow_forward2. Consider the following reactions in open systems: 2H) +Oue) →2H,0) CACO) →CAO +CO18) CaO +SiO) →CaSiO3(s) AgNO) + NaCl → NaNO a) + AgCl) I. 2H2(8) II. 3(s) (s) III. IV. 3(aq) 3(ад) A.mass.of .system , In which of the above could reaction rate be determined by A.time А. I В. II С. II D. IVarrow_forward
- Aluminum forms a tough oxide layer that protects the metal underneath from abrasion and further oxidation. A thin layer of this oxide forms naturally when in contact with air, but with electrochemical anodization one can produce a thicker layer that can significantly extend the lifetime of metal parts. The main form of aluminum oxide is Al2O3, which is a non-conductive network solid. It forms from aluminum via the following series of reactions: Al₂O3 (s) + 6 H+ (aq) + 6 e¯ ⇒ 2 Al (s) + 3 H₂0 (1) H₂ (g) 2 H+ (aq) + 2 e¯ Eº = 0.00 V When exposed to a strong base such as sodium hydroxide, the aluminum oxide can etch away, dissolving: Al₂O3 (s) + 2OH(aq) + 3 H₂O (1) → 2 Al(OH) (aq) You will perform the anodization under acidic conditions, using concentrated sulfuric acid (approximately 18 M) as the electrolyte. Under acidic anodization conditions, aluminum forms first a "barrier layer" of dense oxide coating the electrode, then a thicker and more porous oxide coating (thickness depends on…arrow_forward4 o When burning ethanol in a closed system, which of the following is the best claim and reasoning? The ethanol reaction stops because a bromthymol blue solution that's present turns the same color as when ethanol vapor is blown into the solution. O Water is produced because a bromthymol blue solution that's present turns the same color as when water vapor is blown into the solution. O Evaporated ethanol is produced because it burns. Carbon dioxide is produced because a bromthymol blue solution that's present turns the same color as when carbon dioxide is blown into the solution. 1 2 4. 6 7 8 Next acerarrow_forwardCould someone Please Help Me with this! No Plagirism Please! 3 · Provide at least 1 example of a reversible process (either physical or chemical). What has to be done to the process to make it reverse? Part 2—Copper (II) Chloride System - Cu(H2O)62+(aq) + 4Cl–(aq) + heat ⇆ CuCl42–(aq) + 6H2O(l) Blue Green Test Tube Procedure (Impacts) Predictions Observations (Result/Shift) 1 No tests (control) 2 Add 4M NaCl dropwise until change is observed *increases concentration of Cl─ in the system 3 Add 0.05M AgNO3 drop-wise until change is observed *decreases concentration of Cl─ in the system 4 Add H2O dropwise until change is observed *increases concentration of H2O in the system 5 Add acetone dropwise until change is observed *decreases concentration of H2O in the system 6 Place in hot water bath for 1-2 minutes *adds heat to the reaction system…arrow_forward
- When the following gas reaction reaches equilibrium, at a certain temperature, the moles fractions of the four reactive species satisfy the following relation (we will use "y" for mole fractions since all of these reagents and products are gases): CO+H20 CO2+H2 K= [yCO2][yH2] [yco][yH20] Where K= 1. Assume that the feed introduced to the reactor is 2 mol of CO and 4 mol of H20 only. Also assume that the reaction reaches equilibrium. a) Calculate the extent of reaction in equilibrium (Šeg) b) Calculate the equilibrium composition, for this you need to calculate the mole fraction for all reactants and products.arrow_forwardAt room temperature when HI (aq) is added to potassium bisulfite, KHSO3(s), sulfur dioxide is formed and the reaction vessel becomes cold. HI (aq) + KHSO3(s)→ SO2(g) + H2O(l) + K+(aq) + I-(aq). Fill in the blank: A.) If the temp of the reaction vessel is increased then Ea,rev will... (decrease, stay the same, increase, or need more info). B.) If the temp of the reaction vessel is increased then Kc will... (decrease, stay the same, increase, or need more info).arrow_forwardWhen the following gas reaction reaches equilibrium, at a certain temperature, the moles fractions of the four reactive species satisfy the following relation (we will use "y" for mole fractions since all of these reagents and products are gases): CO+H20 > CO2+H2 [yCO2][yH2] K= [yC0][YH20] Where K= 1. Assume that the feed introduced to the reactor is 2 mol of CO and 4 mol of H20 only. Also assume that the reaction reaches equilibrium. need to calculate the mole fraction for all a) Calculate the equilibrium composition, for this reactants and products. Answer: yCo2 = 2/9 = 2/9 yH2 УСо - 1/9 YH2O = 4/9|arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





