
Concept explainers
A hydrogen atom in the organic base pyridine, C5H5N, can be substituted by various atoms or groups to give XC5H4N, where X is an atom such as Cl or a group such as CH3. The following table gives Ka values for the conjugate acids of a variety of substituted pyridines.
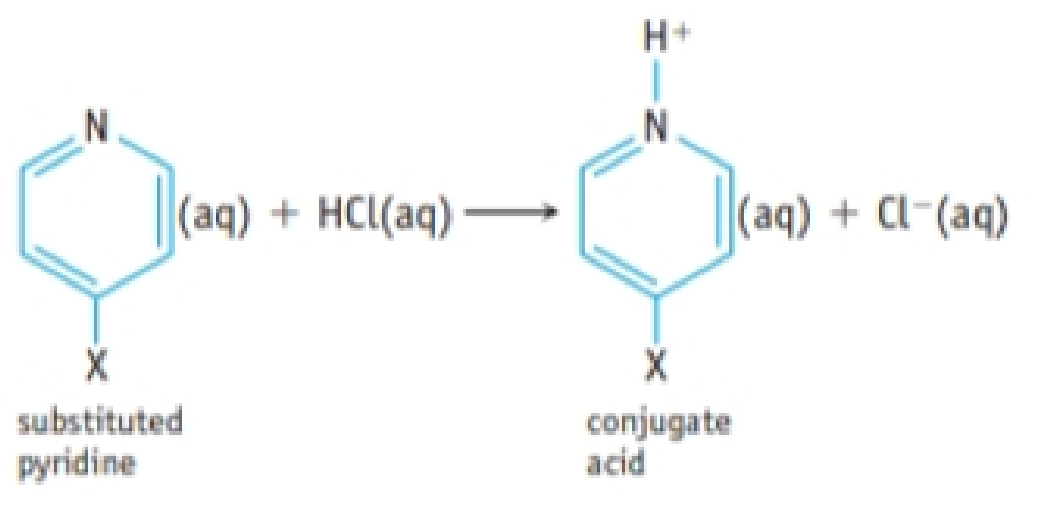
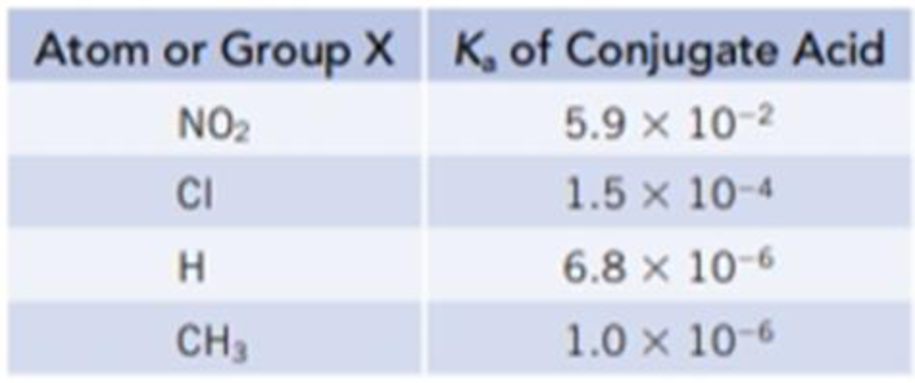
- (a) Suppose each conjugate acid is dissolved in sufficient water to give a 0.050 M solution. Which solution would have the highest pH? The lowest pH?
- (b) Which of the substituted pyridines is the strongest Brønsted base? Which is the weakest Brønsted base?
(a)
Interpretation:
The conjugate acid which have the highest
Concept Introduction:
The
Higher the value of
Answer to Problem 114IL
The solution containing
Explanation of Solution
An equilibrium constant
For Any acid HA,
The relative strength of an acid and base in water can be also expressed quantitatively with an equilibrium constant as follows:
An equilibrium constant
Given:
The
Initial Concentration of each solution is
Set up an ICE table for the reaction of
Substitute the values in equation (2) to calculate
Substitute the value of
The value of
Substitute the value of hydronium ion concentration in equation (1) to calculate the value of
Therefore, the value of
Similarly, Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
The solution containing
(b)
Interpretation:
Strongest
Concept Introduction:
A conjugate acid-base pair contains two compounds that differ only by a hydrogen ion and a charge of
The stronger the acid, the weaker its conjugate base and vice-verca. That is, the larger the values of
Answer to Problem 114IL
The strongest bronsted base is
Explanation of Solution
The dissociation of
A small value of
The dissociation of
A large value of
The strongest bronsted base is
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry & Chemical Reactivity
- Trimethylamine, (CH3)3N, reacts readily with diborane, B2H6. The diborane dissociates to two BH3 fragments, each of which can react with trimethylamine to form a complex, (CH3)3N:BH3. Write an equation for this reaction and interpret it in terms of Lewis acid-base theory.arrow_forwardPure liquid ammonia ionizes in a manner similar to that of water. (a) Write the equilibrium for the autoionization of liquid ammonia. (b) Identify the conjugate acid form and the base form of the solvent. (c) Is NaNH2 an acid or a base in this solvent? (d) Is ammonium bromide an acid or a base in this solvent?arrow_forwardThe following reactions illustrate Brnsted acid-base behavior. Complete each equation. a.HI(aq)+?H3O+(aq)+I(aq) b.NH3(l)+?NH4++NH2 c.H2C2O4(aq)+H2O(l)?+HC2O4(aq) d.H2N2O2(aq)+H2O(l)H3O+(aq)+? e.?+H2O(l)H3O+(aq)+CO32(aq)arrow_forward
- 1. Which of the following can act as a Lewis acid? (Hint : In each case, draw the Lewis electron dot structure of the molecule or ion. Are there lone pairs of electrons on the central atom? If so, it can be a Lewis base. Does the central atom lack an electron pair? If so, it can behave as a Lewis acid.) PH3 BCl3 H2S HS−arrow_forwardWhich of the following compounds or ions has the weakest conjugate base? Briefly explain your choice. a) HCN b) HClO c) NH4+arrow_forwardWrite equations that show NH3 as both a conjugate acid and a conjugate base.arrow_forward
- Place the species in each of the following groups in order of increasing acid strength. a. H2O, H2S, H2Se (bond energies: HO, 467 kJ/mol; HS, 363 kJ/mol; HSe, 276 kJ/mol) b. CH3CO2H, FCH2CO2H, F2CHCO2H, F3CCO2H c. NH4+, HONH3+ d. NH4+, PH4+ (bond energies: NH, 391 kJ/mol; PH, 322 kJ/mol) Give reasons for the orders you chose.arrow_forwardConsider the following four biological solutions: (1) bile, pH 8.0, (2) blood, pH 7.4, (3) urine, pH 6.0, and (4) gastric juice, pH 1.6. a. Which solution has the lowest [H3O+]? b. Which solution has the lowest [OH]? c. List the solutions in order of decreasing acidity. d. List the solutions in order of increasing basicity.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





