
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 117IL
Sulfanilic acid, which is used in making dyes, is made by reacting aniline with sulfuric acid.
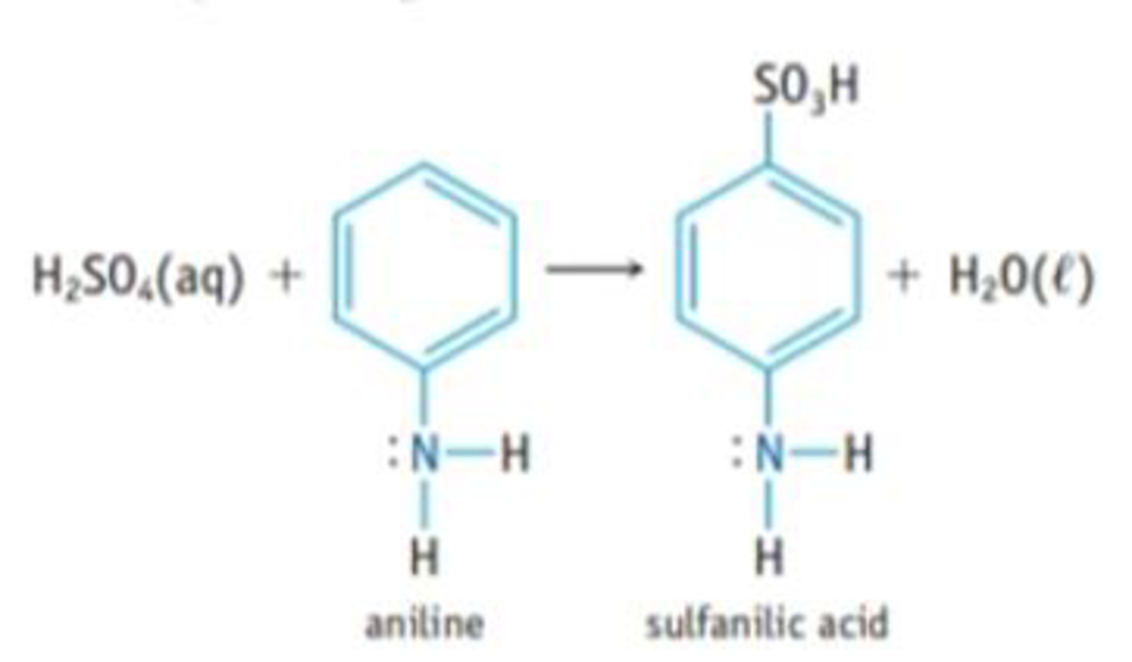
- (a) Is aniline a Brønsted base, a Lewis base, or both? Explain, using its possible reactions with HCl, BF3, or other acid.
- (b) Sulfanilic acid has a pKa value of 3.23. The sodium salt of the acid, Na(H2NC6H4SO3), is quite soluble in water. If you dissolve 1.25 g of the salt in water to give 125 ml, of solution, what is the pH of the solution?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Another way to express acid strength is by using pk b:pK a =−logK a Another way to express base strength is by using pK b pK b =−logK b A new potential heart medicine, code-named X-281, is being tested by a pharmaceutical company, Pharma-pill. As a research technician at Pharma-pill, you are told that X-281 is a monoprotic weak acid, but because of security concerns, the actual chemical formula must remain top secret. The company is interested in the drug's K a value because only the dissociated form of the chemical is active in preventing cholesterol buildup in arteries.To find the pK a of X-281, you prepare a 0.079 M M test solution of X-281 at 25.0 ∘ C The pH of the solution is determined to be 3.00. What is the pK a of X-281?
Define pKa for a weak acid. What is the relationshipbetween the value of the pKa and the strength of theacid? Do the same for a weak base.
Phenylacetic acid (C6H5CH2COOH, simplified here to HPAc is a monoprotic acid. It builds up in the blood of persons with phenylketonuria, that if untreated, causes mental retardation and death. A study of the acid shows that the pH of 0.120 M HPAc is 2.62. What are (a) the Ka and (b) pKa of phenylacetic acid?
Chapter 16 Solutions
Chemistry & Chemical Reactivity
Ch. 16.2 - What are the hydronium ion and hydroxide ion...Ch. 16.4 - For each of the following salts in water, predict...Ch. 16.5 - (a) Which is the stronger Bronsted acid, HCO3 or...Ch. 16.7 - A solution prepared from 0.055 mol of butanoic...Ch. 16.7 - What are the equilibrium concentrations of acetic...Ch. 16.7 - What are the equilibrium concentrations of HF, F...Ch. 16.7 - The weak base, CIO (hypochlorite ion), is used in...Ch. 16.7 - Calculate the pH after mixing 15 mL of 0.12 M...Ch. 16.8 - What is the pH of a 0.10 M solution of oxalic...Ch. 16.10 - Prob. 1.1ACP
Ch. 16.10 - Prob. 1.2ACPCh. 16.10 - The pKa, of the conjugate acid of atropine is...Ch. 16.10 - Convert the pK values to K values for the...Ch. 16.10 - Other solvents also undergo autoionization. (a)...Ch. 16.10 - Prob. 2.3ACPCh. 16.10 - Prob. 2.4ACPCh. 16.10 - To measure the relative strengths of bases...Ch. 16 - Write the formula and the give the name of the...Ch. 16 - Write the formula and give the name of the...Ch. 16 - What are the products of each of the following...Ch. 16 - What are the products of each of the following...Ch. 16 - Write balanced equations showing how the hydrogen...Ch. 16 - Write a balanced equation showing how the HPO42...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - An aqueous solution has a pH of 3.75. What is the...Ch. 16 - A saturated solution of milk of magnesia. Mg(OH)2,...Ch. 16 - What is the pH of a 0.0075 M solution of HCl? What...Ch. 16 - What is the pH of a 1.2 104 M solution of KOH?...Ch. 16 - What is the pH of a 0.0015 M solution of Ba(OH)2?Ch. 16 - The pH of a solution of Ba(OH)2 is 10.66 at 25 ....Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Several acids are listed here with their...Ch. 16 - Several acids are listed here with their...Ch. 16 - Which of the following ions or compounds has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ion has the...Ch. 16 - Dissolving K2CO3 in water gives a basic solution....Ch. 16 - Dissolving ammonium bromide in water gives an...Ch. 16 - If each of the salts listed here were dissolved in...Ch. 16 - Which of the following common food additives gives...Ch. 16 - Prob. 27PSCh. 16 - Prob. 28PSCh. 16 - Prob. 29PSCh. 16 - An organic acid has pKa = 8.95. What is its Ka...Ch. 16 - Prob. 31PSCh. 16 - Which is the stronger of the following two acids?...Ch. 16 - Chloroacetic acid (ClCH2CO2H) has Ka = 1.41 103....Ch. 16 - A weak base has Kb = 1.5 109. What is the value...Ch. 16 - The trimethylammonium ion, (CH3)3NH+, is the...Ch. 16 - The chromium(III) ion in water, [Cr(H2O)6]3+. Is a...Ch. 16 - Acetic acid and sodium hydrogen carbonate, NaHCO3,...Ch. 16 - Ammonium chloride and sodium dihydrogen phosphate,...Ch. 16 - For each of the following reactions, predict...Ch. 16 - For each of the following reactions, predict...Ch. 16 - Equal molar quantities of sodium hydroxide and...Ch. 16 - Equal molar quantities of hydrochloric acid and...Ch. 16 - Equal molar quantities of acetic acid and sodium...Ch. 16 - Equal molar quantities of ammonia and sodium...Ch. 16 - A 0.015 M solution of hydrogen cyanate, HOCN, has...Ch. 16 - A 0.10 M solution of chloroacetic acid, CICH2CO2H,...Ch. 16 - A 0.025 M solution of hydroxyl amine has a pH of...Ch. 16 - Methylamine, CH3NH2, is a weak base. CH3NH2(aq) +...Ch. 16 - A 2.5 103 M solution of an unknown acid has a pH...Ch. 16 - A 0.015M solution of a base has a pH of 10.09 a)...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - The ionizations constant of a very weak acid, HA...Ch. 16 - What are the equilibrium concentration of H3O+, CN...Ch. 16 - Phenol (C6H5OH) commonly called carbolic acid is a...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - A hypothetical weak base has Kb=5.0104.Calculate...Ch. 16 - The weak base methylamine, CH3NH2, has Kb=4.2104....Ch. 16 - Calculate the pH of a 0.12 M aqueous solution of...Ch. 16 - Calculate the pH of a 0.0010 M aqueous solution of...Ch. 16 - A solution of hydrofluoric acid, HF, has a pH of...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Sodium cyanide is the salt of the weak acid HCN....Ch. 16 - The sodium salt of propionic acid, NaCH3CH2CO2 is...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and the...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - Oxalic acid, H2C2O4, is a diprotic acid. Write a...Ch. 16 - Sodium carbonate is a diprotic base. Write a...Ch. 16 - Prove that Ka1 Kb2 = Kw for oxalic acid H2C2O4,...Ch. 16 - Prove that Ka3 Kb1 = Kw for phosphoric acid,...Ch. 16 - Sulphurous acid, H2SO3, is a weak acid capable of...Ch. 16 - Ascorbic acid (vitamin C, C6H8O6) is a diprotic...Ch. 16 - Hydrazine, N2H4, can interact with water in two...Ch. 16 - Ethylene diamine, H2NCH2CH2NH2, can interact with...Ch. 16 - Which should be stronger acid, HOCN or HCN?...Ch. 16 - Prob. 78PSCh. 16 - Explain why benzene sulfonic acid is a Brnsted...Ch. 16 - The structure of ethylene diamine is illustrated...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Carbon monoxide forms complexes with low-valent...Ch. 16 - Trimethylamine, (CH3)3N, is a common reagent. It...Ch. 16 - About this time, you may be wishing you had an...Ch. 16 - Consider the following ions: NH4+, CO32, Br, S2,...Ch. 16 - A 2.50 g sample of a solid that could be Ba(OH)2...Ch. 16 - In a particular solution, acetic acid is 11%...Ch. 16 - Hydrogen, H2S, and sodium acetate, NaCH3CO2 are...Ch. 16 - For each of the following reactions predict...Ch. 16 - A monoprotic acid HX has Ka = 1.3 103. Calculate...Ch. 16 - Arrange the following 0.10M solutions in order of...Ch. 16 - m-Nitrophenol, a weak acid, can be used as a pH...Ch. 16 - The butylammonium ion, C4H9NH3+, has a Ka of 2.3 ...Ch. 16 - The local anaesthetic novocaine is the hydrogen...Ch. 16 - Pyridine is weak organic base and readily forms a...Ch. 16 - The base ethylamine (CH3CH2NH2) has a Kb of. A...Ch. 16 - Chloroacetic acid, ClCH2CO2H, is a moderately weak...Ch. 16 - Saccharin (HC7H4NO3S) is a weak acid with pKa =...Ch. 16 - Given the following solutions: (a) 0.1 M NH3 (b)...Ch. 16 - For each of the following salts, predict whether a...Ch. 16 - Nicotine, C10H14N2, has two basic nitrogen atoms...Ch. 16 - Prob. 103GQCh. 16 - The equilibrium constant for the reaction of...Ch. 16 - The equilibrium constant for the reaction of...Ch. 16 - Calculate the pH of the solution that results from...Ch. 16 - To what volume should 1.00 102 mL of any weak...Ch. 16 - The hydrogen phthalate ion, C8HsO4, is a weak acid...Ch. 16 - Prob. 109GQCh. 16 - Prob. 110GQCh. 16 - Prob. 111ILCh. 16 - Prob. 112ILCh. 16 - Prob. 113ILCh. 16 - A hydrogen atom in the organic base pyridine,...Ch. 16 - Nicotinic acid, C6H5NO2, is found in minute...Ch. 16 - Prob. 116ILCh. 16 - Sulfanilic acid, which is used in making dyes, is...Ch. 16 - Amino acids are an important group of compounds....Ch. 16 - How can water be both a Brnsied base and a Lewis...Ch. 16 - The nickel(II) ion exists as [Ni(H2O)4]2+ in...Ch. 16 - The halogens form three stable, weak acids, HOX....Ch. 16 - The acidity of the oxoacids was described in...Ch. 16 - Perchloric acid behaves as an acid, even when it...Ch. 16 - You purchase a bottle of water. On checking its...Ch. 16 - Prob. 125SCQCh. 16 - Prob. 126SCQCh. 16 - Prob. 127SCQCh. 16 - Prob. 128SCQCh. 16 - Consider a salt of a weak base and a weak acid...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider these acids (a) Arrange the acids in order of increasing acid strength from weakest to strongest. (b) Which acid has the smallest pKa value?arrow_forwardFind the value of Kb for the conjugate base of the following organic acids. (a) picric acid used in the manufacture of explosives; Ka = 0.16 (b) trichloroacetic acid used in the treatment of warts; Ka = 0.20arrow_forwardWeak base B has a pKb of 6.78 and weak acid HA has a pKa of 5.12. a Which is the stronger base, B or A? b Which is the stronger acid, HA or BH+? c Consider the following reaction: B(aq)+HA(aq)BH+(aq)+A(aq) Based on the information about the acid/base strengths for the species in this reaction, is this reaction favored to proceed more to the right or more to the left? Why? d An aqueous solution is made in which the concentration of weak base B is one half the concentration of its acidic salt, BHCl, where BH+ is the conjugate weak add of B. Calculate the pH of the solution. e An aqueous solution is made in which the concentration of weak acid HA twice the concentration of the sodium salt of the weak acid, NaA. Calculate the pH of the solution. f Assume the conjugate pairs B/BH+ and HA/A are capable of being used as color-based end point indicators in acidbase titrations, where B is the base form indicator and BH is the acid form indicator, and HA is the acid form indicator and A is the base form indicator. Select the indicator pair that would be best to use in each of the following titrations: (1) Titration of a strong acid with a strong base. (i) B/BH+ (ii) HA/A (2) Titration of a weak base with a strong acid. (i) B/BH+ (ii) HA/Aarrow_forward
- Calculate the pKa of the weak acid HA, given that a solution that is 0.357 in HA and 1.24 in A- has pH = 5.32.Provide your answer rounded to 2 decimal digits.arrow_forwardI need help with this problem. Compare the pH of propionic acid and nitric acid when they are dissolved in water at a concentration of 20 mM using the "Arrhenius Concept". Include:a balanced equation describing the acid-base reactions involvedThe calculation of the pKa for bothThe calculation of the two pH's (of propionic acid and nitric acid when they are dissolved in water at a concentration of 20 mM)and A calculation of the percent of dissociation of both propionic acid and nitric acid.The gibbs free energy ΔG are listed as the following:Nitric acid: -152 kJ/molPropionic Acid : -291 kJ/molPropionate: -361 kJ/molarrow_forwardGiven that acetic acid has Ka = 1.8 x 10–5, what is the pH of a solution that contains the molar ratio of conjugate base-to-acid: [CH3CO2–]/[CH3CO2H] = 10/1?arrow_forward
- Benozic acid (C6H5COOH) has a pKa of 4.20. a. What is the ratio of the acid to its conjugate base at a pH of 2.20?arrow_forwardAcidified potassium bromate(V), KBrO3, reacts with hydrogen sulfide, H2S, to give a yellow solid and an orange solution. On shaking the solution with trichloroethane, the trichloroethane layer turned orange-red. (a) Suggest the identity of yellow solid and the orange solution. Describe the type of reaction taking place. (b) Construct a balanced equation for the reaction between KBrO3 and H2S. (C) BrO3- is the conjugate base of HBrO3. Draw the dot and cross structure of HBrO3. (d) HBr is a stronger acid than HCl. However, HBrO3 is a weaker acid than HCIO3. Explain the difference in acidities.arrow_forwardThe following table shows the structures of some organic molecules together with their pKa values.Write the ionization reaction, they must take into account the hydrogen that is given off, the hydrogen acid. What is the strongest acid? Propose an explanation to justify the difference in acidity values.arrow_forward
- What is the pH of a vinegar with 25.65 % (w/v) acetic acid in water? The pKa of acetic acid is 4.497. Record your answer with 2 decimals.arrow_forwardDetermine the pKa of an acid; a 4e-3M solution of which has a pH of 2.4arrow_forwardWhat is the pH of a solution that contains 0.135 M HCOOH and 0.215 M HCOONa?The pKa of formic acid is 3.750 What is the pH of the solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Ocean Chemistry; Author: Beverly Owens;https://www.youtube.com/watch?v=IDQzklIr57Q;License: Standard YouTube License, CC-BY