
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 122SCQ
The acidity of the oxoacids was described in Section 16.9, and a larger number of acids are listed in the table below.
- (a) What general trends do you see in these data?
- (b) What has a greater effect on acidity, the number of O atoms bonded directly to the central atom E or the number of OH groups?
- (c) Look at the acids based on Cl, N, and S. Is there a correlation of acidity with the formal charge on the central atom, E?
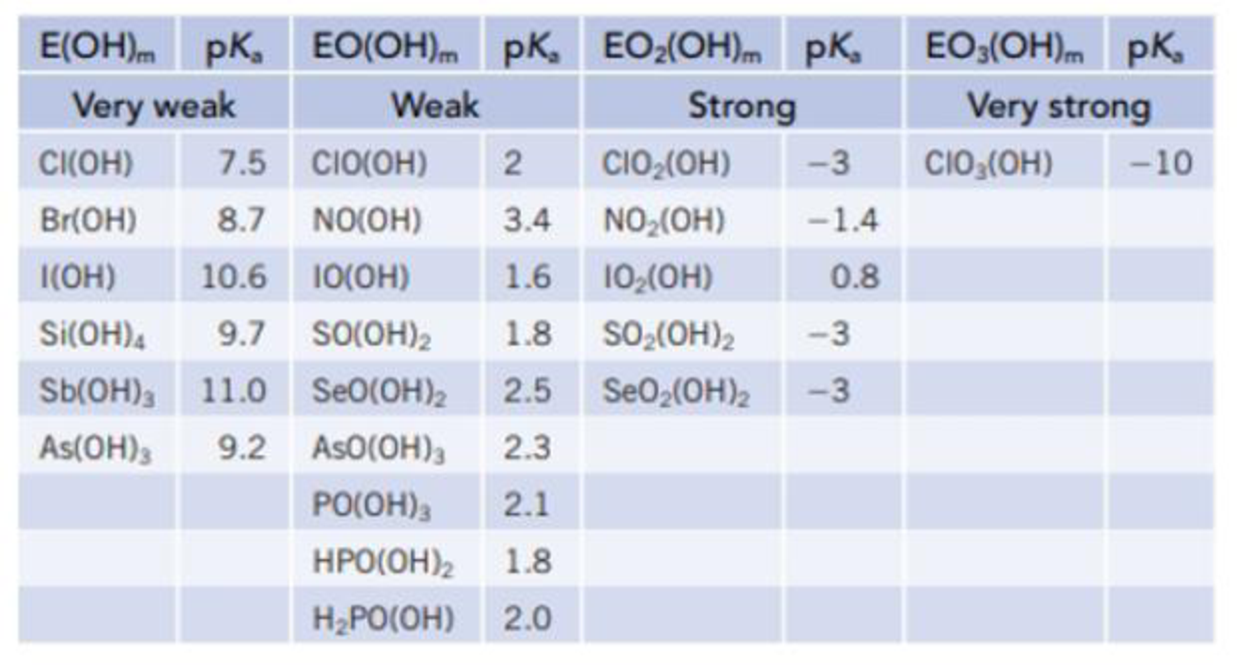
- (d) The acid H3PO3 has a pKa of 1.8, and this led to some insight into its structure. If the structure of the acid were P(OH)3, what would be its predicted pKa value? Given that this is a diprotic acid, which H atoms are lost as H+ ions?
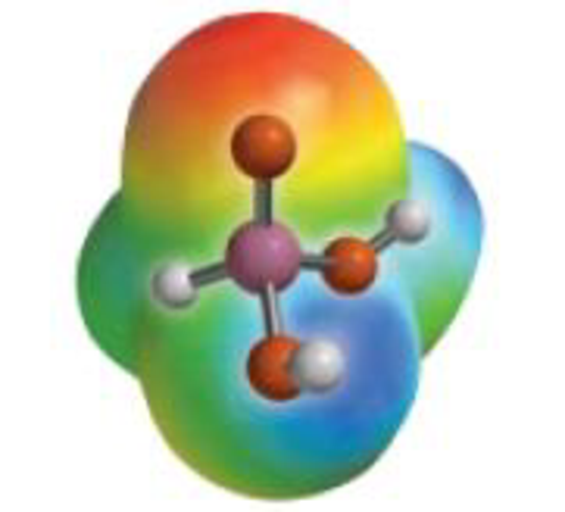
The odd H3PO3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Chemistry & Chemical Reactivity
Ch. 16.2 - What are the hydronium ion and hydroxide ion...Ch. 16.4 - For each of the following salts in water, predict...Ch. 16.5 - (a) Which is the stronger Bronsted acid, HCO3 or...Ch. 16.7 - A solution prepared from 0.055 mol of butanoic...Ch. 16.7 - What are the equilibrium concentrations of acetic...Ch. 16.7 - What are the equilibrium concentrations of HF, F...Ch. 16.7 - The weak base, CIO (hypochlorite ion), is used in...Ch. 16.7 - Calculate the pH after mixing 15 mL of 0.12 M...Ch. 16.8 - What is the pH of a 0.10 M solution of oxalic...Ch. 16.10 - Prob. 1.1ACP
Ch. 16.10 - Prob. 1.2ACPCh. 16.10 - The pKa, of the conjugate acid of atropine is...Ch. 16.10 - Convert the pK values to K values for the...Ch. 16.10 - Other solvents also undergo autoionization. (a)...Ch. 16.10 - Prob. 2.3ACPCh. 16.10 - Prob. 2.4ACPCh. 16.10 - To measure the relative strengths of bases...Ch. 16 - Write the formula and the give the name of the...Ch. 16 - Write the formula and give the name of the...Ch. 16 - What are the products of each of the following...Ch. 16 - What are the products of each of the following...Ch. 16 - Write balanced equations showing how the hydrogen...Ch. 16 - Write a balanced equation showing how the HPO42...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - An aqueous solution has a pH of 3.75. What is the...Ch. 16 - A saturated solution of milk of magnesia. Mg(OH)2,...Ch. 16 - What is the pH of a 0.0075 M solution of HCl? What...Ch. 16 - What is the pH of a 1.2 104 M solution of KOH?...Ch. 16 - What is the pH of a 0.0015 M solution of Ba(OH)2?Ch. 16 - The pH of a solution of Ba(OH)2 is 10.66 at 25 ....Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Several acids are listed here with their...Ch. 16 - Several acids are listed here with their...Ch. 16 - Which of the following ions or compounds has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ion has the...Ch. 16 - Dissolving K2CO3 in water gives a basic solution....Ch. 16 - Dissolving ammonium bromide in water gives an...Ch. 16 - If each of the salts listed here were dissolved in...Ch. 16 - Which of the following common food additives gives...Ch. 16 - Prob. 27PSCh. 16 - Prob. 28PSCh. 16 - Prob. 29PSCh. 16 - An organic acid has pKa = 8.95. What is its Ka...Ch. 16 - Prob. 31PSCh. 16 - Which is the stronger of the following two acids?...Ch. 16 - Chloroacetic acid (ClCH2CO2H) has Ka = 1.41 103....Ch. 16 - A weak base has Kb = 1.5 109. What is the value...Ch. 16 - The trimethylammonium ion, (CH3)3NH+, is the...Ch. 16 - The chromium(III) ion in water, [Cr(H2O)6]3+. Is a...Ch. 16 - Acetic acid and sodium hydrogen carbonate, NaHCO3,...Ch. 16 - Ammonium chloride and sodium dihydrogen phosphate,...Ch. 16 - For each of the following reactions, predict...Ch. 16 - For each of the following reactions, predict...Ch. 16 - Equal molar quantities of sodium hydroxide and...Ch. 16 - Equal molar quantities of hydrochloric acid and...Ch. 16 - Equal molar quantities of acetic acid and sodium...Ch. 16 - Equal molar quantities of ammonia and sodium...Ch. 16 - A 0.015 M solution of hydrogen cyanate, HOCN, has...Ch. 16 - A 0.10 M solution of chloroacetic acid, CICH2CO2H,...Ch. 16 - A 0.025 M solution of hydroxyl amine has a pH of...Ch. 16 - Methylamine, CH3NH2, is a weak base. CH3NH2(aq) +...Ch. 16 - A 2.5 103 M solution of an unknown acid has a pH...Ch. 16 - A 0.015M solution of a base has a pH of 10.09 a)...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - The ionizations constant of a very weak acid, HA...Ch. 16 - What are the equilibrium concentration of H3O+, CN...Ch. 16 - Phenol (C6H5OH) commonly called carbolic acid is a...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - A hypothetical weak base has Kb=5.0104.Calculate...Ch. 16 - The weak base methylamine, CH3NH2, has Kb=4.2104....Ch. 16 - Calculate the pH of a 0.12 M aqueous solution of...Ch. 16 - Calculate the pH of a 0.0010 M aqueous solution of...Ch. 16 - A solution of hydrofluoric acid, HF, has a pH of...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Sodium cyanide is the salt of the weak acid HCN....Ch. 16 - The sodium salt of propionic acid, NaCH3CH2CO2 is...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and the...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - Oxalic acid, H2C2O4, is a diprotic acid. Write a...Ch. 16 - Sodium carbonate is a diprotic base. Write a...Ch. 16 - Prove that Ka1 Kb2 = Kw for oxalic acid H2C2O4,...Ch. 16 - Prove that Ka3 Kb1 = Kw for phosphoric acid,...Ch. 16 - Sulphurous acid, H2SO3, is a weak acid capable of...Ch. 16 - Ascorbic acid (vitamin C, C6H8O6) is a diprotic...Ch. 16 - Hydrazine, N2H4, can interact with water in two...Ch. 16 - Ethylene diamine, H2NCH2CH2NH2, can interact with...Ch. 16 - Which should be stronger acid, HOCN or HCN?...Ch. 16 - Prob. 78PSCh. 16 - Explain why benzene sulfonic acid is a Brnsted...Ch. 16 - The structure of ethylene diamine is illustrated...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Carbon monoxide forms complexes with low-valent...Ch. 16 - Trimethylamine, (CH3)3N, is a common reagent. It...Ch. 16 - About this time, you may be wishing you had an...Ch. 16 - Consider the following ions: NH4+, CO32, Br, S2,...Ch. 16 - A 2.50 g sample of a solid that could be Ba(OH)2...Ch. 16 - In a particular solution, acetic acid is 11%...Ch. 16 - Hydrogen, H2S, and sodium acetate, NaCH3CO2 are...Ch. 16 - For each of the following reactions predict...Ch. 16 - A monoprotic acid HX has Ka = 1.3 103. Calculate...Ch. 16 - Arrange the following 0.10M solutions in order of...Ch. 16 - m-Nitrophenol, a weak acid, can be used as a pH...Ch. 16 - The butylammonium ion, C4H9NH3+, has a Ka of 2.3 ...Ch. 16 - The local anaesthetic novocaine is the hydrogen...Ch. 16 - Pyridine is weak organic base and readily forms a...Ch. 16 - The base ethylamine (CH3CH2NH2) has a Kb of. A...Ch. 16 - Chloroacetic acid, ClCH2CO2H, is a moderately weak...Ch. 16 - Saccharin (HC7H4NO3S) is a weak acid with pKa =...Ch. 16 - Given the following solutions: (a) 0.1 M NH3 (b)...Ch. 16 - For each of the following salts, predict whether a...Ch. 16 - Nicotine, C10H14N2, has two basic nitrogen atoms...Ch. 16 - Prob. 103GQCh. 16 - The equilibrium constant for the reaction of...Ch. 16 - The equilibrium constant for the reaction of...Ch. 16 - Calculate the pH of the solution that results from...Ch. 16 - To what volume should 1.00 102 mL of any weak...Ch. 16 - The hydrogen phthalate ion, C8HsO4, is a weak acid...Ch. 16 - Prob. 109GQCh. 16 - Prob. 110GQCh. 16 - Prob. 111ILCh. 16 - Prob. 112ILCh. 16 - Prob. 113ILCh. 16 - A hydrogen atom in the organic base pyridine,...Ch. 16 - Nicotinic acid, C6H5NO2, is found in minute...Ch. 16 - Prob. 116ILCh. 16 - Sulfanilic acid, which is used in making dyes, is...Ch. 16 - Amino acids are an important group of compounds....Ch. 16 - How can water be both a Brnsied base and a Lewis...Ch. 16 - The nickel(II) ion exists as [Ni(H2O)4]2+ in...Ch. 16 - The halogens form three stable, weak acids, HOX....Ch. 16 - The acidity of the oxoacids was described in...Ch. 16 - Perchloric acid behaves as an acid, even when it...Ch. 16 - You purchase a bottle of water. On checking its...Ch. 16 - Prob. 125SCQCh. 16 - Prob. 126SCQCh. 16 - Prob. 127SCQCh. 16 - Prob. 128SCQCh. 16 - Consider a salt of a weak base and a weak acid...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is a structural formula for imidazole, a building block of the essential amino acid histidine (Chapter 27). It is also a building block of histamine, a compound all too familiar to people with allergies and takers of antihistamines. When imidazole is dissolved in water, proton transfer to it gives a cation. Is this cation better represented by structure A or B? Explain.arrow_forwardThe following reactions illustrate Brnsted acid-base behavior. Complete each equation. a.HI(aq)+?H3O+(aq)+I(aq) b.NH3(l)+?NH4++NH2 c.H2C2O4(aq)+H2O(l)?+HC2O4(aq) d.H2N2O2(aq)+H2O(l)H3O+(aq)+? e.?+H2O(l)H3O+(aq)+CO32(aq)arrow_forward1. Which of the following can act as a Lewis acid? (Hint : In each case, draw the Lewis electron dot structure of the molecule or ion. Are there lone pairs of electrons on the central atom? If so, it can be a Lewis base. Does the central atom lack an electron pair? If so, it can behave as a Lewis acid.) PH3 BCl3 H2S HS−arrow_forward
- Hydrazine, N2H4 (having the structure H2NNH2), and its derivatives have been used as rocket fuels. Draw the Lewis electron-dot formula for the hydrazine molecule. Describe the geometries expected about the nitrogen atoms in this molecule. Why would you expect hydrazine to be basic? Which substance, NH3 or N2H4, would you expect to be more basic? Why? Write the chemical equation in which hydrazine reacts with hydrochloric acids to form the salt N2H5Cl. Consider the positive ion of this salt. How does its basic character compare with that of NH3 and N2H4? Explain.arrow_forwardCoal and other fossil fuels usually contain sulfur compounds that produce sulfur dioxide, SO2, when burned. One possible way to remove the sulfur dioxide is to pass the combustion gases into a tower packed with calcium oxide, CaO. Write the equation for the reaction. Identify each reactant as either a Lewis acid or a Lewis base. Explain how you arrived at your answer.arrow_forwardAluminum chloride, AlCl3, behaves more as a molecular compound than an ionic one. This is illustrated in its ability to form a fourth covalent bond with a chloride ion: AlCl3+ClAlCl4. From the Lewis diagram of the aluminum chloride molecule and the electron configuration of the chloride ion, show that this is an acidbase reaction in the Lewis sense, and identify the Lewis acid and the Lewis base. Aluminum chloride is a white solid at room conditions, although it is typically manufactured mixed with some yellow ironIII chloride. Its appearance is that of an ionic compound.arrow_forward
- How do the components of a conjugate acid—base pair differ from one another4? Give an example of a conjugate acid—base pair to illustrate your answer.arrow_forwardArsenic acid (H3AsO4) is a moderately weak triprotic acid. Write equations showing its stepwise dissociation. Which of the three anions formed in these reactions will be the strongest Brnsted base? Which will be the weakest Brnsted base? Explain your answers.arrow_forwardPyruvic acid, which is produced in metabolic reactions, has the structure Would you predict that this acid is a mono-, di-, tri-, or tetraprotic acid? Give your reasoning.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY