
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 74PS
Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 6.8 × 10−5 and Ka2 = 2.7 × 10−12). What is the pH of a solution that contains 5.0 mg of acid per millilitre of solution?
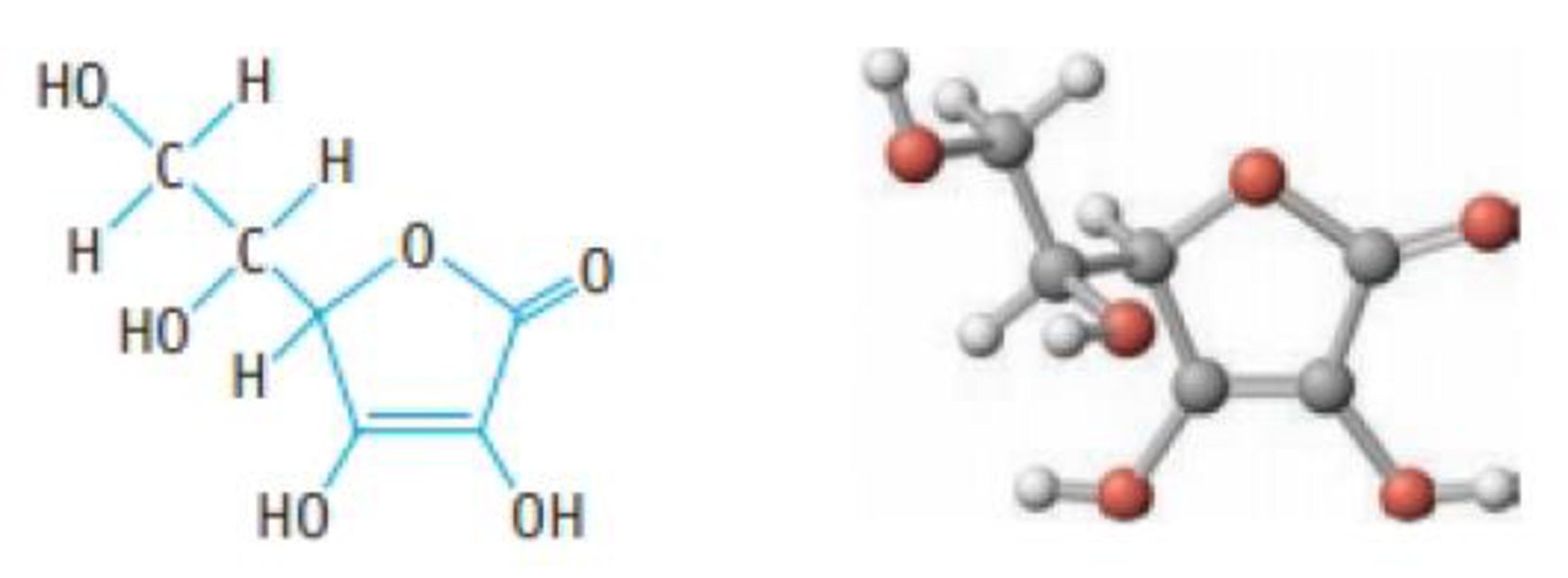
Ascorbic acid
Expert Solution & Answer
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

schedule09:05
Chapter 16 Solutions
Chemistry & Chemical Reactivity
Ch. 16.2 - What are the hydronium ion and hydroxide ion...Ch. 16.4 - For each of the following salts in water, predict...Ch. 16.5 - (a) Which is the stronger Bronsted acid, HCO3 or...Ch. 16.7 - A solution prepared from 0.055 mol of butanoic...Ch. 16.7 - What are the equilibrium concentrations of acetic...Ch. 16.7 - What are the equilibrium concentrations of HF, F...Ch. 16.7 - The weak base, CIO (hypochlorite ion), is used in...Ch. 16.7 - Calculate the pH after mixing 15 mL of 0.12 M...Ch. 16.8 - What is the pH of a 0.10 M solution of oxalic...Ch. 16.10 - Prob. 1.1ACP
Ch. 16.10 - Prob. 1.2ACPCh. 16.10 - The pKa, of the conjugate acid of atropine is...Ch. 16.10 - Convert the pK values to K values for the...Ch. 16.10 - Other solvents also undergo autoionization. (a)...Ch. 16.10 - Prob. 2.3ACPCh. 16.10 - Prob. 2.4ACPCh. 16.10 - To measure the relative strengths of bases...Ch. 16 - Write the formula and the give the name of the...Ch. 16 - Write the formula and give the name of the...Ch. 16 - What are the products of each of the following...Ch. 16 - What are the products of each of the following...Ch. 16 - Write balanced equations showing how the hydrogen...Ch. 16 - Write a balanced equation showing how the HPO42...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - In each of the following acid-base reactions,...Ch. 16 - An aqueous solution has a pH of 3.75. What is the...Ch. 16 - A saturated solution of milk of magnesia. Mg(OH)2,...Ch. 16 - What is the pH of a 0.0075 M solution of HCl? What...Ch. 16 - What is the pH of a 1.2 104 M solution of KOH?...Ch. 16 - What is the pH of a 0.0015 M solution of Ba(OH)2?Ch. 16 - The pH of a solution of Ba(OH)2 is 10.66 at 25 ....Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Write an equilibrium constant expression for the...Ch. 16 - Several acids are listed here with their...Ch. 16 - Several acids are listed here with their...Ch. 16 - Which of the following ions or compounds has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ions has the...Ch. 16 - Which of the following compounds or ion has the...Ch. 16 - Dissolving K2CO3 in water gives a basic solution....Ch. 16 - Dissolving ammonium bromide in water gives an...Ch. 16 - If each of the salts listed here were dissolved in...Ch. 16 - Which of the following common food additives gives...Ch. 16 - Prob. 27PSCh. 16 - Prob. 28PSCh. 16 - Prob. 29PSCh. 16 - An organic acid has pKa = 8.95. What is its Ka...Ch. 16 - Prob. 31PSCh. 16 - Which is the stronger of the following two acids?...Ch. 16 - Chloroacetic acid (ClCH2CO2H) has Ka = 1.41 103....Ch. 16 - A weak base has Kb = 1.5 109. What is the value...Ch. 16 - The trimethylammonium ion, (CH3)3NH+, is the...Ch. 16 - The chromium(III) ion in water, [Cr(H2O)6]3+. Is a...Ch. 16 - Acetic acid and sodium hydrogen carbonate, NaHCO3,...Ch. 16 - Ammonium chloride and sodium dihydrogen phosphate,...Ch. 16 - For each of the following reactions, predict...Ch. 16 - For each of the following reactions, predict...Ch. 16 - Equal molar quantities of sodium hydroxide and...Ch. 16 - Equal molar quantities of hydrochloric acid and...Ch. 16 - Equal molar quantities of acetic acid and sodium...Ch. 16 - Equal molar quantities of ammonia and sodium...Ch. 16 - A 0.015 M solution of hydrogen cyanate, HOCN, has...Ch. 16 - A 0.10 M solution of chloroacetic acid, CICH2CO2H,...Ch. 16 - A 0.025 M solution of hydroxyl amine has a pH of...Ch. 16 - Methylamine, CH3NH2, is a weak base. CH3NH2(aq) +...Ch. 16 - A 2.5 103 M solution of an unknown acid has a pH...Ch. 16 - A 0.015M solution of a base has a pH of 10.09 a)...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - The ionizations constant of a very weak acid, HA...Ch. 16 - What are the equilibrium concentration of H3O+, CN...Ch. 16 - Phenol (C6H5OH) commonly called carbolic acid is a...Ch. 16 - What are the equilibrium concentrations of...Ch. 16 - A hypothetical weak base has Kb=5.0104.Calculate...Ch. 16 - The weak base methylamine, CH3NH2, has Kb=4.2104....Ch. 16 - Calculate the pH of a 0.12 M aqueous solution of...Ch. 16 - Calculate the pH of a 0.0010 M aqueous solution of...Ch. 16 - A solution of hydrofluoric acid, HF, has a pH of...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Sodium cyanide is the salt of the weak acid HCN....Ch. 16 - The sodium salt of propionic acid, NaCH3CH2CO2 is...Ch. 16 - Calculate the hydronium ion concentration and pH...Ch. 16 - Calculate the hydronium ion concentration and the...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - For each of the following cases, decide whether...Ch. 16 - Oxalic acid, H2C2O4, is a diprotic acid. Write a...Ch. 16 - Sodium carbonate is a diprotic base. Write a...Ch. 16 - Prove that Ka1 Kb2 = Kw for oxalic acid H2C2O4,...Ch. 16 - Prove that Ka3 Kb1 = Kw for phosphoric acid,...Ch. 16 - Sulphurous acid, H2SO3, is a weak acid capable of...Ch. 16 - Ascorbic acid (vitamin C, C6H8O6) is a diprotic...Ch. 16 - Hydrazine, N2H4, can interact with water in two...Ch. 16 - Ethylene diamine, H2NCH2CH2NH2, can interact with...Ch. 16 - Which should be stronger acid, HOCN or HCN?...Ch. 16 - Prob. 78PSCh. 16 - Explain why benzene sulfonic acid is a Brnsted...Ch. 16 - The structure of ethylene diamine is illustrated...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Decide whether each of the following substances...Ch. 16 - Carbon monoxide forms complexes with low-valent...Ch. 16 - Trimethylamine, (CH3)3N, is a common reagent. It...Ch. 16 - About this time, you may be wishing you had an...Ch. 16 - Consider the following ions: NH4+, CO32, Br, S2,...Ch. 16 - A 2.50 g sample of a solid that could be Ba(OH)2...Ch. 16 - In a particular solution, acetic acid is 11%...Ch. 16 - Hydrogen, H2S, and sodium acetate, NaCH3CO2 are...Ch. 16 - For each of the following reactions predict...Ch. 16 - A monoprotic acid HX has Ka = 1.3 103. Calculate...Ch. 16 - Arrange the following 0.10M solutions in order of...Ch. 16 - m-Nitrophenol, a weak acid, can be used as a pH...Ch. 16 - The butylammonium ion, C4H9NH3+, has a Ka of 2.3 ...Ch. 16 - The local anaesthetic novocaine is the hydrogen...Ch. 16 - Pyridine is weak organic base and readily forms a...Ch. 16 - The base ethylamine (CH3CH2NH2) has a Kb of. A...Ch. 16 - Chloroacetic acid, ClCH2CO2H, is a moderately weak...Ch. 16 - Saccharin (HC7H4NO3S) is a weak acid with pKa =...Ch. 16 - Given the following solutions: (a) 0.1 M NH3 (b)...Ch. 16 - For each of the following salts, predict whether a...Ch. 16 - Nicotine, C10H14N2, has two basic nitrogen atoms...Ch. 16 - Prob. 103GQCh. 16 - The equilibrium constant for the reaction of...Ch. 16 - The equilibrium constant for the reaction of...Ch. 16 - Calculate the pH of the solution that results from...Ch. 16 - To what volume should 1.00 102 mL of any weak...Ch. 16 - The hydrogen phthalate ion, C8HsO4, is a weak acid...Ch. 16 - Prob. 109GQCh. 16 - Prob. 110GQCh. 16 - Prob. 111ILCh. 16 - Prob. 112ILCh. 16 - Prob. 113ILCh. 16 - A hydrogen atom in the organic base pyridine,...Ch. 16 - Nicotinic acid, C6H5NO2, is found in minute...Ch. 16 - Prob. 116ILCh. 16 - Sulfanilic acid, which is used in making dyes, is...Ch. 16 - Amino acids are an important group of compounds....Ch. 16 - How can water be both a Brnsied base and a Lewis...Ch. 16 - The nickel(II) ion exists as [Ni(H2O)4]2+ in...Ch. 16 - The halogens form three stable, weak acids, HOX....Ch. 16 - The acidity of the oxoacids was described in...Ch. 16 - Perchloric acid behaves as an acid, even when it...Ch. 16 - You purchase a bottle of water. On checking its...Ch. 16 - Prob. 125SCQCh. 16 - Prob. 126SCQCh. 16 - Prob. 127SCQCh. 16 - Prob. 128SCQCh. 16 - Consider a salt of a weak base and a weak acid...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of a solution obtained by mixing 235 mL of NaOH with a pH of 11.57 and 316 mL of Sr(OH)2 with a pH of 12.09? Assume that volumes are additive.arrow_forwardIndicate whether each of the following samples is acidic, basic, or neutral. a. butter, pH 6.1 b. lemon juice, pH 2.2 c. peach, pH 3.5 d. milk of magnesia, pH 10.5arrow_forwardA typical vitamin C tablet (containing pure ascorbic acid, H2C6H6O6) weighs 500. mg. One vitamin C tablet is dissolved in enough water to make 200.0 mL of solution. Calculate the pH of this solution. Ascorbic acid is a diprotic acid.arrow_forward
- Consider 50.0 mL of a solution of weak acid HA (Ka = 1.00 106), which has a pH of 4.000. What volume of water must be added to make the pH = 5.000?arrow_forwardThe base ethylamine (CH3CH2NH2) has a Kb of. A closely related base, ethanolamine(HOCH2CH2NH2), has a Kb of 3.2105. (a) Which of the two bases is stronger? (b) Calculate the pH of a 0.10M solution of the strong base?arrow_forwardNicotinic acid, C6H5NO2, is found in minute amounts in all living cells, hut appreciable amounts occur in liver, yeast, milk, adrenal glands, white meat, and corn. Whole wheat (lour contains about 60. 0g per gram of flour. One gram (1.00 g) of the acid dissolves in water to give 60. mL of solution having a pH of 2.70. What is the approximate value of Ka for the acid? Nicotinic acidarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY