
Concept explainers
Find the charge on each of the capacitors in Figure P16.43.
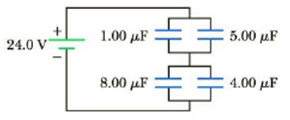
Figure P16.43
Answer to Problem 43P
The charge on
The charge on
The charge on
The charge on
Explanation of Solution
The capacitors
The capacitors
The capacitances
Therefore,
Substitute
Formula to calculate the total charge is,
Substitute
Formula to calculate the charge on
Substitute
Formula to calculate the charge on
Substitute
Formula to calculate the charge on
Substitute
Formula to calculate the charge on
Substitute
Conclusion:
The charge on
The charge on
The charge on
The charge on
Want to see more full solutions like this?
Chapter 16 Solutions
College Physics
- Four capacitors are connected as shown in Figure P16.48. (a) Find the equivalent capacitance between points a and b. (b) Calculate the charge on each capacitor, taking Vab = 15.0 V. Figure P16.48arrow_forward(a) Find the equivalent capacitance between points a and b for the group of capacitors connected as shown in Figure P20.44. Take C1 = 5.00 F, C2 = 10.0 F, and C3 = 2.00 F. (b) What charge is stored on C3 if the potential difference between points a and b is 60.0 V? Figure P20.44arrow_forwardFour capacitors are connected as shown in Figure P16.48. (a) Find the equivalent capacitance between points a and b. (b) Calculate the charge on each capacitor, taking Vab = 15.0 V. Figure P16.48arrow_forward
- Four capacitors are connected as shown in Figure P25.11. (a) Find the equivalent capacitance between points a and b. (b) Calculate the charge on each capacitor, taking Vab = 15.0 V. Figure P25.11arrow_forwardFor the system of capacitors shown in Figure P16.41, find (a) the equivalent capacitance of the system, (b) the charge on each capacitor, and (c) the potential difference across each capacitor. Figure P16.41 Problems 41 and 60.arrow_forwardFind the equivalent capacitance between points a and b in the combination of capacitors shown in Figure P25.13. Figure P25.13arrow_forward
- Four capacitors are connected as shown in Figure P20.45. (a) Find the equivalent capacitance between points a and b. (b) Calculate the charge on each capacitor, taking Vab = 15.0 V. Figure P20.45arrow_forwardFind (a) the equivalent capacitance of the capacitors in Figure P26.26, (b) the charge on each capacitor, and (c) the potential difference across each capacitor.arrow_forwardWhat If? The two capacitors of Problem 13 (C1 = 5.00 F and C2 = 12.0 F) are now connected in series and to a 9.00-Y battery. Find (a) the equivalent capacitance of the combination. (b) the potential difference across each capacitor, and (c) the charge on each capacitor.arrow_forward
- For the system of capacitors shown in Figure P16.41, find (a) the equivalent capacitance of the system, (b) the charge on each capacitor, and (c) the potential difference across each capacitor. Figure P16.41 Problems 41 and 60.arrow_forward(a) Find the equivalent capacitance between points a and b for the group of capacitors connected as shown in Figure P25.12 (page 686). Take C1 = 5.00 F, C2 = 10.0 F, and C3 = 2.00 F. (b) What charge is stored on C3 if the potential difference between points a and b is 60.0 V? Figure P25.12arrow_forward(a) Find the equivalent capacitance between points a and b for the group of capacitors connected as shown in Figure P16.46 if C1 = 5.00 F, C2 = 10.00 F, and C3 = 2.00 F. (b) If the potential between points a and b is 60.0 V, what charge is stored on C5? Figure P16.46arrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





