
(a)
Interpretation:
The Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atoms.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around
symbol of element to satisfy the octet (or duplet) for each atom. - Add charge on overall structure in case of polytatomic cation or anion.
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Here,
(a)
Explanation of Solution
Thus total valence electrons is sum of the valence electrons for each atom along with uni-positive in
Hence, 5 pairs are allocated to form Lewis structure of

The formal charge on each atom in the Lewis structure can be calculated from the equation as follows:
Substitute 5for
Substitute 6for
Hence
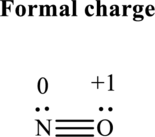
(b)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Thus total valence electrons is sum of the valence electrons on each atom in
Hence, of these five pairs two are allocated as lone pairs and three pairs are added so as to form triple bond to obtain Lewis structure of

The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Substitute 5for
Hence each
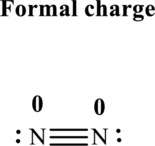
(c)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Thus total valence electron is sum of the valence electrons for each atom in
Hence, 5 pairs are allocated to form Lewis structure of

The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Substitute 4for
Substitute 6 for
Hence
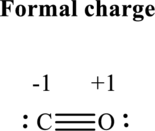
(d)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Thus total valence electron is sum of the valence electrons for each atom in
Hence, 5 pairs are arranged to form Lewis structure of
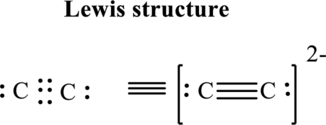
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Substitute 4 for
Hence each
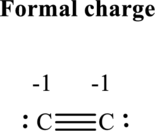
(e)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(e)
Explanation of Solution
Thus total valence electron is sum of the valence electrons for each atom in
Hence, 5 pairs are arranged to form Lewis structure of
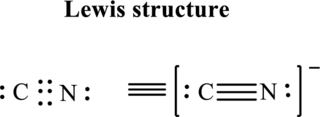
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Substitute 4 for
Substitute 5 for
Hence formal charge on
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- What is the formal charge on the central chlorine atom in the molecular ion [ClO4]- ? Assume that all of the Cl-O bonds are single bonds.arrow_forwardWhat is the formal charge on the chlorine (Cl) atom in PCl₆⁻?arrow_forwardDraw the Lewis structures of cyanate (OCN-) and fulminate (CNO-) ions and calculate their formal charges. Discuss their stability by giving resonance structures.arrow_forward
- How many resonance structures can be drawn for the hydrogen tellurate ion (HTeO4–) in which the central tellurium atom bears a –1 formal charge and the oxygens bear formal charges of either zero or –1? Enter your answer as a whole number.arrow_forwardDraw the Lewis structure with lowest formal charges, anddetermine the charge of each atom in (a) BF₄⁻; (b) ClNO.arrow_forwardWrite a Lewis structure for HC2─ and assign any formal charges to the correct atom.arrow_forward
- Determine the number of valence electrons in SO₃ and then draw the corresponding Lewis structure (with minimized formal charges).arrow_forwardHow many non-bonding electrons are on the central atom in the optimized Lewis structure of NBr3, in which the formal charges are minimized?arrow_forwardWrite the Lewis structures for CH2N2, including all resonance forms, and show formal charges.arrow_forward
- the best Lewis structure for the fulminate ion, CNO–, what is the formal charge on the central nitrogen atom?arrow_forwardDraw the Lewis structure of SOCl₄ (with minimized formal charges) and then determine if the molecule is polar or nonpolar.arrow_forwardFind the Lewis structures of CO32- and O3 molecules showing their formal charges and Draw the resonance structures. (6C and 8O)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

