
Concept explainers
(a)
Interpretation:
The Lewis structure of
Concept Introduction:
Valence Shell Electron Pair Repulsion model predicts shape by inclusion of bond angles and most distant arrangement of atoms that leads to minimum repulsion.
For molecules that have lone pairs around central atom, lone pairs influence shape, because there are no atoms at the positions occupied by these lone pairs. The key rule that governs the molecular shape, in this case, is the extent of lone pair–lone pair repulsions are far greater than lone bond pair or bond pair-bond pair repulsions. The table that summarized the molecular shapes possible for various combinations of bonded and lone pairs are given as follows:
(a)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 15 electron pairs are assigned as lone pairs of each of the
Hence, the Lewis structure
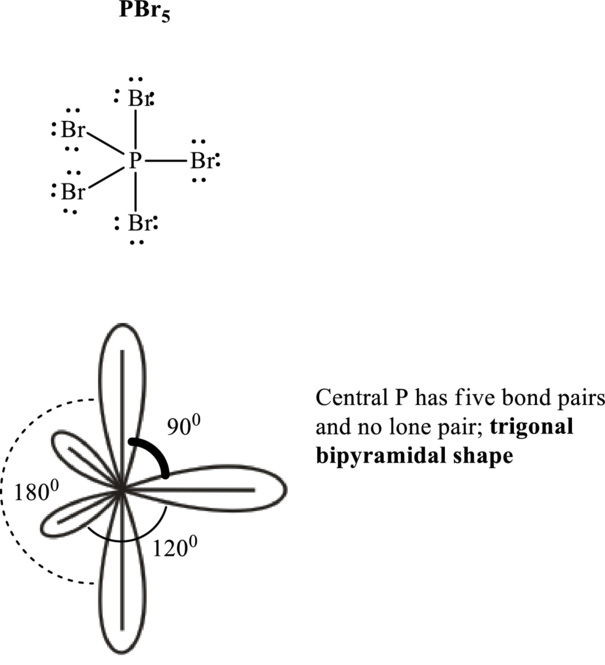
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any trigonal pyramidal geometry the VSEPR formula is predicted as
It is evident that in
The bond angles are
(b)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 11 electron pairs are allotted as lone pairs of each of the fluorine, oxygen atoms and central xenon to satisfy respective octets. Thus, the Lewis structure and corresponding VSEPR geometry
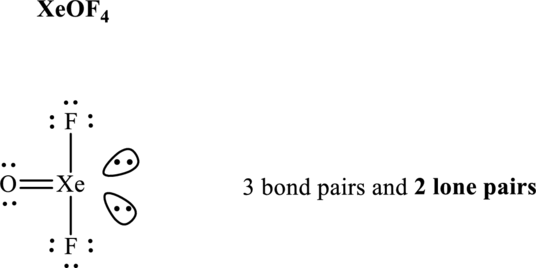
It is evident that in
(c)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each chlorine and central iodine in
The skeleton structure in
These 15 electron pairs are allotted as lone pairs to each of the
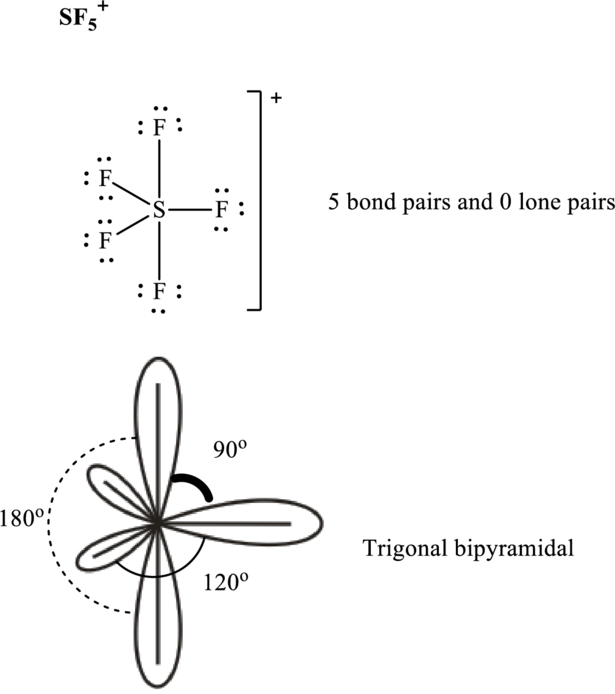
It is evident that in
(d)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each
The skeleton structure in
These 11 electron pairs are allotted as lone pairs of each of the fluorine atoms and central iodine to satisfy respective octets. Hence, the Lewis structure and corresponding VSPER geometry in
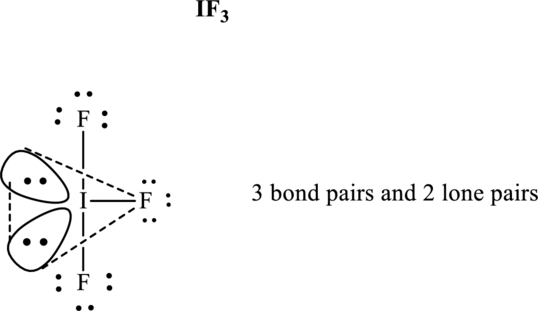
It is evident that in
Lone pairs tend to occupy the equatorial locations of trigonal plane so that they are
(e)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 10 electron pairs are allotted as lone pairs or multiple bonds to satisfy respective octets. Hence, the Lewis structure and corresponding VSPER geometry in
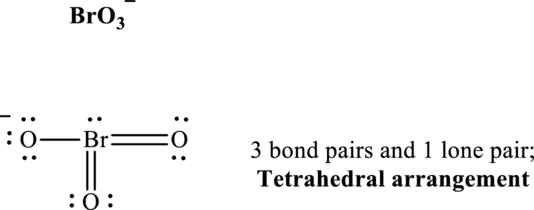
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any see-saw species the VSEPR formula is predicted as
The bond pairs in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- It is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardThe cations O2+ and N2+ are formed when molecules of O2 and N2 are subjected to intense, high-energy solar radiation in Earths upper atmosphere. Write the electron configuration for O2+. Predict its bond order and magnetic behavior.arrow_forwardDraw the Lewis structure of BrF₃ and then determine the ideal bonding angle(s) of the central atom.arrow_forward
- How many non-bonding electrons are on the central atom in the optimized Lewis structure of NBr3, in which the formal charges are minimized?arrow_forwardDetermine the number of valence electrons in sulfuric acid (H₂SO₄) and then draw the corresponding Lewis structure (with minimized formal charges).arrow_forwardThere are three possible structures for PCl2F3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.arrow_forward
- Draw the Lewis structure of HClO₃ (with minimized formal charges) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forwardDraw the Lewis dot structure and calculate the formal charges of the oxygen and phosphorus atoms of PO4 3- . What is the molecular geometry of the molecule?arrow_forwardDraw the Lewis structure for acetamide (CH3CONH2), an organic compound, and determine the geometry about each interior atom. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. Which resonance structure can account for the planar geometry about the nitrogen atom?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



