
Concept explainers
(a)
Interpretation:
Valence shell electronic configuration of
Concept Introduction:
Molecular orbital diagram is a linear combination of atomic orbitals of similar energy and similar symmetry. It is formed by the proper overlap of the atomic orbitals.
There are 3 types of molecular orbitals as follows:
1. Bonding molecular orbital: They are formed by the constructive interference of atomic orbitals and electrons in it stabilize the molecule and are of lesser in energy.
2. Antibonding molecular orbital: This type of orbitals increases the energy of molecule and destabilizes it and weakens the bond between the atoms.
3. Non-bonding molecular orbital: These types of orbitals have energy similar to atomic orbitals that is addition or removal of electron does not change the energy of molecule.
The order of energy in molecular orbital follows two rules as follows:
1. For
2. For atomic number more than 14 order of energy is,
(a)
Explanation of Solution
For
The symbol for nitrogen is
One positive charge is added up in the total valence count.
Thus total valence electrons is sum of the valence electrons for each atom in
Hence, 9 electrons are to be arranged in each molecular orbital to obtain an electronic configuration. Since number of electrons in
Hence, the electronic configuration of
For
The symbol for nitrogen is
Two positive charges are subtracted from the total valence count.
Thus total valence electrons are sum of the valence electrons for each atom in
Hence, 8 electrons are to be arranged in each molecular orbital to obtain an electronic configuration. Since number of electrons in
Hence, the electronic configuration of
For
The symbol for nitrogen is
Two negative charges are added up in total valence count.
Thus total valence electrons are sum of the valence electrons for each atom in
Hence, 12 electrons are to be arranged in each molecular orbital to obtain an electronic configuration. Since number of electrons in
Hence, the electronic configuration of
(b)
Interpretation:
The bond order of
Concept Introduction:
Bond order
(b)
Explanation of Solution
For
The electronic configuration of
Substitute 7 for number of electrons in bonding orbitals and 2 for number of electrons in antibonding orbitals in equation (1) to calculate bond order.
Hence, the bond order of the molecule
For
The electronic configuration of
Substitute 6 for number of electrons in bonding orbitals and 2 for number of electrons in antibonding orbitals in equation (1) to calculate bond order.
Hence, the bond order of the molecule
For
The electronic configuration of
Substitute 8 for number of electrons in bonding orbitals and 4 for number of electrons in antibonding orbitals in equation (1) to calculate bond order.
Hence, the bond order of the molecule
(c)
Interpretation:
Molecular orbital diagram of
Concept Introduction:
Refer to (a)
(c)
Explanation of Solution
For
The electronic configuration of
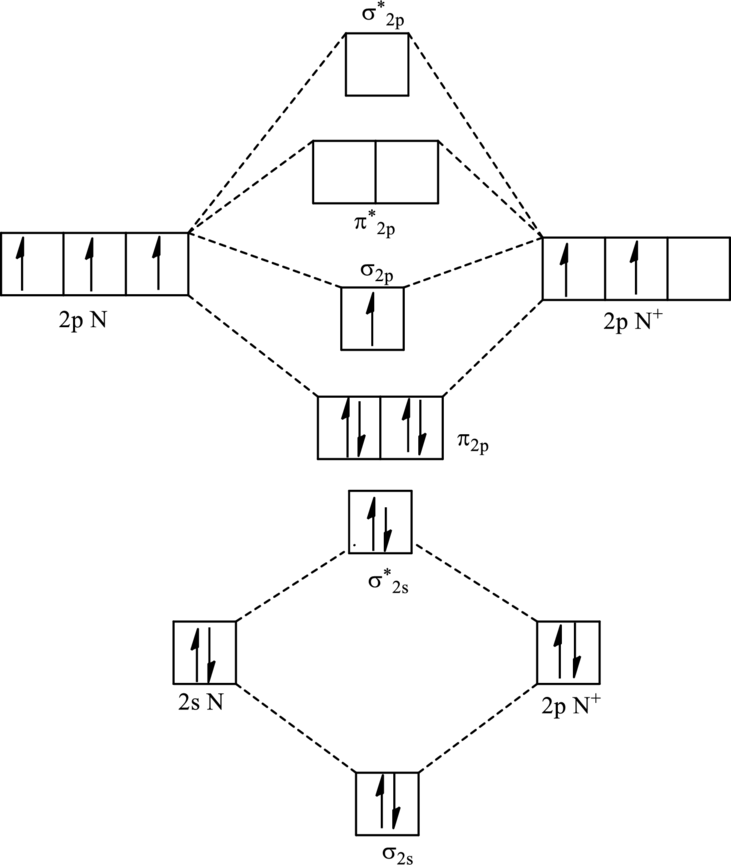
Since
For
The electronic configuration of
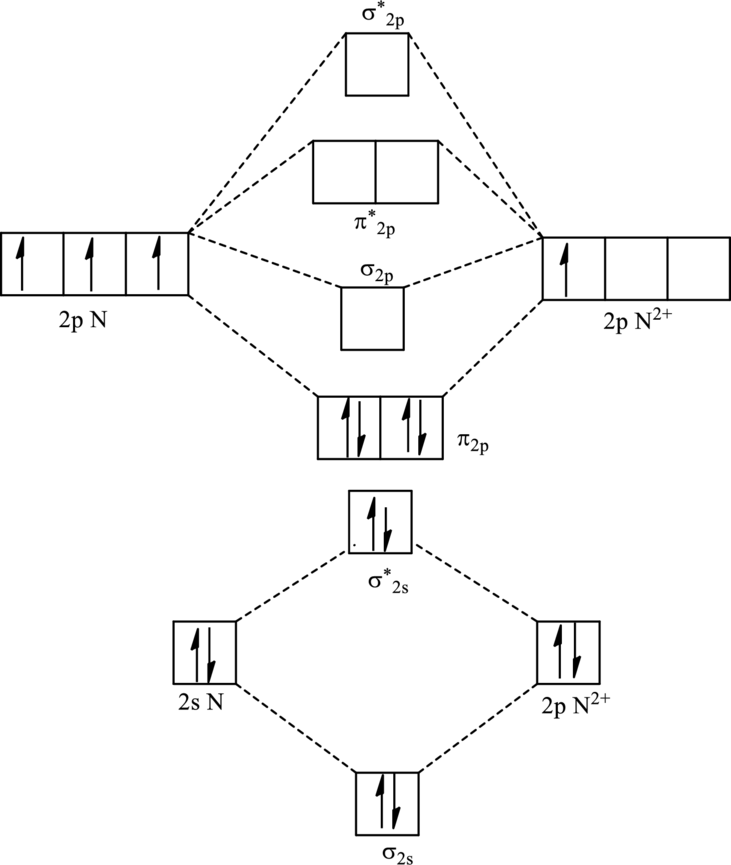
Since
For
The electronic configuration of
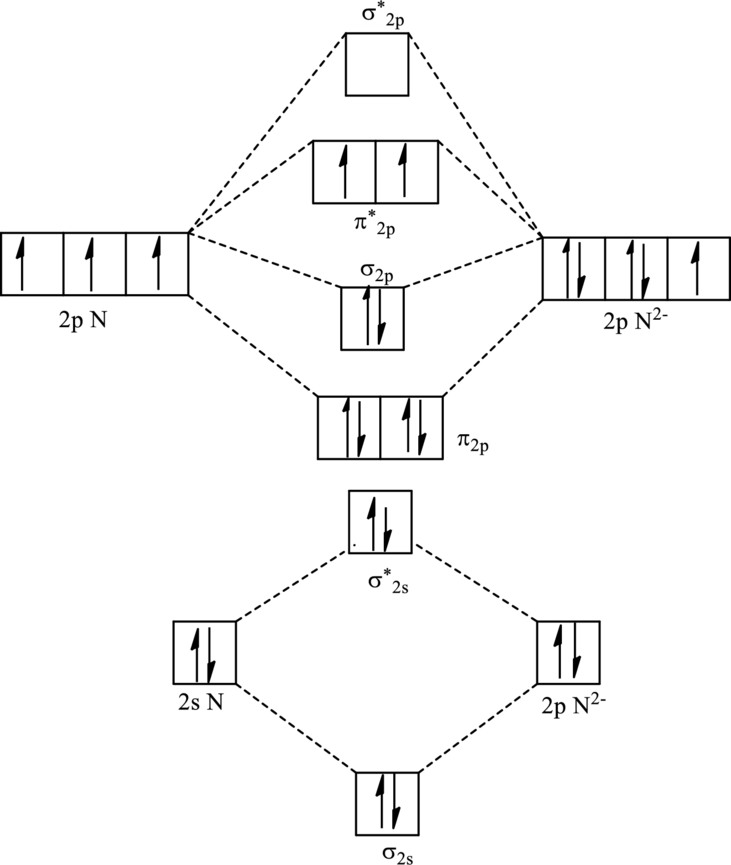
Since
(d)
Interpretation:
Character of highest energy orbital of
Concept Introduction:
Refer to (a)
(d)
Explanation of Solution
For
The electronic configuration of
For
The electronic configuration of
For
The electronic configuration of
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Write the ground-state electron configuration of O2 and calculate the bond order.arrow_forwardWhat would be the electron configuration for a HeH- molecular ion? What bond order would you predict? How stable should such a species be?arrow_forwardPlease explain the scientific method with few words and write the lewis structure of PCl3, then indicate the molecular geometry of the molecule according to VSEPR and indicate the hybridization type of the structurearrow_forward
- The existence of compounds of the noble gases was once a great surprise and stimulated a great deal of theoretical work. Sketch the molecular orbital energy level diagram for XeF and deduce its ground state electron configurations. Is XeF likely to have a shorter bond length than XeF+?arrow_forwardWhich would have a higher CO stretching frequency, Cr(CO)6 or V(CO)6–? Explain why in detail using molecular orbital arguments.arrow_forwardFor a N H 4 + ion, identify its molecular shape, bond angle, and hybrid orbitals.arrow_forward
- Describe the bonding in the C2 2- ion in terms of molecular orbital theory and compare the bond order to that of C2.arrow_forwardCholesterol, C27H46O, has only one pi bond. With no additional information, what else can you say about its structure?arrow_forward2. Use molecular orbital (MO) theory in description of the following molecules: Which of the following species has the greater bond enthalpy? These chemical species have the similar orbital structure to that of N2. CO, CO+arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



