
Concept explainers
(a)
Interpretation:
Lewis structure of periodate ion
Concept Introduction:
Lewis structure represents covalent bonds and describes valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- A single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around
symbol of element to satisfy the octet (or duplet) for each atom. - Add charge on overall structure in case of polyatomic cation or anion.
(a)
Explanation of Solution
The molecule
The symbol for oxygen is
The symbol for iodine is
One negative charge on molecule is added up as one valence electron in total count.
Thus total valence electrons are sum of the valence electrons for each atom in
The skeleton structure in
To complete the valence electrons of iodine, it forms double bond with three oxygen atoms.
Hence, the Lewis structure for
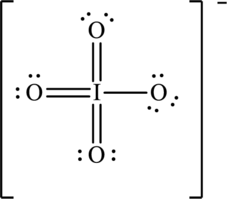
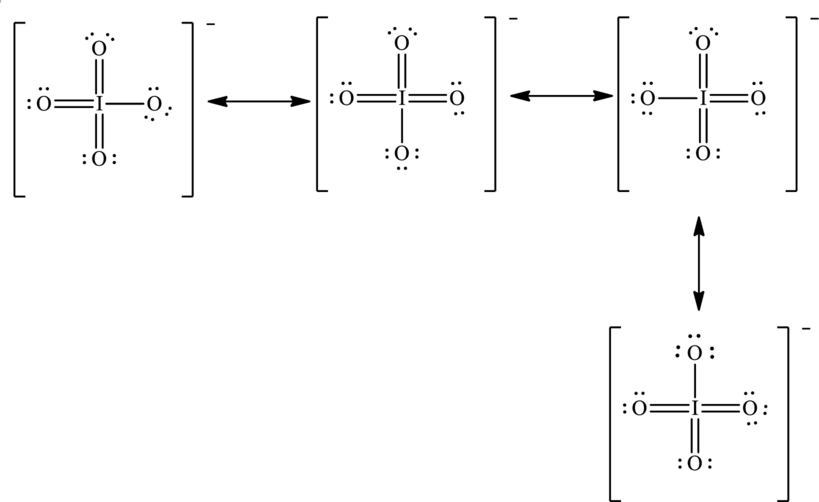
To complete the octet of iodine atom the negative charge is delocalized on each oxygen atom and since there are four oxygen atoms, therefore, four resonance structures will be formed and possible resonance structures are as follows:
(b)
Interpretation:
Lewis structure of hydrogen phosphate ion
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Hydrogen phosphate ion
The symbol for oxygen is
The symbol for hydrogen is
The symbol for phosphorus is
Two negative charges on molecule are added up as one valence electron in total count.
Thus total valence electrons are sum of the valence electrons for each atom in
The skeleton structure in
To complete the valence electrons of phosphorous, it forms double bond with one oxygen atom.
Hence, 20 electrons are allocated as 10 lone pairs on remaining oxygen atoms to complete their octet. The Lewis structure is as follows:
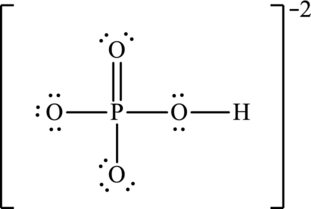
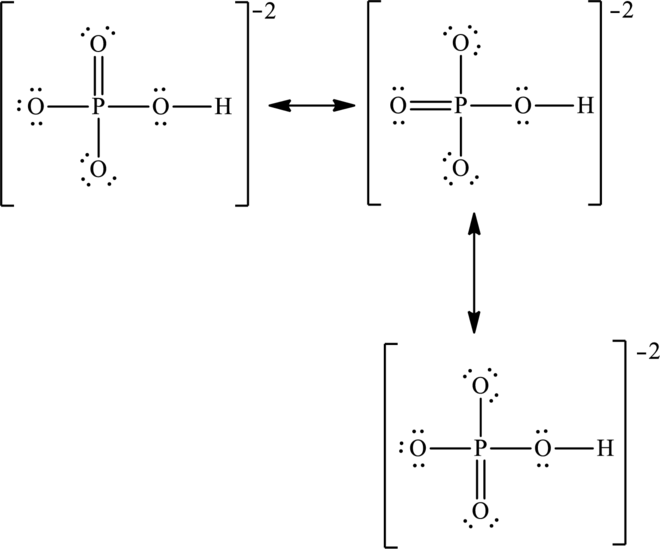
The negative charge is delocalized on three oxygen atoms and therefore three resonance structures will be formed and possible resonance structures are as follows:
(c)
Interpretation:
Lewis structure of chloric acid
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Chloric acid
The symbol for oxygen is
The symbol for hydrogen is
The symbol for chlorine is
Thus total valence electrons are sum of the valence electrons for each atom in
The skeleton structure in
To complete the valence electrons of chlorine, it forms double bond with two oxygen atoms.
Hence, 14 electrons are allocated as 6 lone pairs on remaining oxygen atoms and 1 lone pair on chlorine to complete their respective octet. The Lewis structure is as follows:
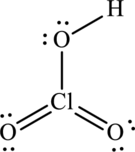
The lone pair on oxygen atom that is attached to chlorine participates in resonance with that and produces
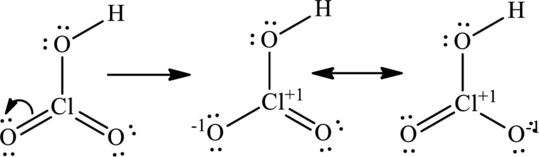
(d)
Interpretation:
Lewis structure of arsenate ion
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Arsenate ion
The symbol for oxygen is
The symbol for arsenic is
The symbol for chlorine is
Three negative charges on molecule is added up as three valence electrons in total count. Thus total valence electrons are sum of the valence electrons for each atom in arsenate ion
The skeleton structure in arsenate ion
To complete the valence electrons of arsenic, it forms double bond with one oxygen atom.
Hence, 22 electrons are allocated as 11 lone pairs on remaining oxygen atoms to complete their octet. The Lewis structure is as follows:
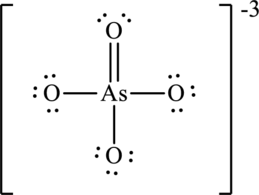
The negative charge is delocalized on each oxygen atom and since there are four oxygen atoms, therefore, four resonance structures will be formed and possible resonance structures are as,
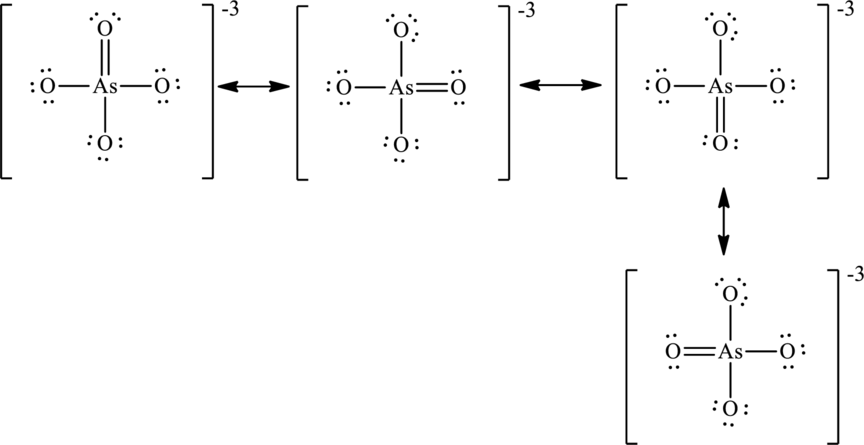
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Several Lewis structures can be written for perbromate ion, , the central Br with all single Br—O bonds, or with one, two, or three Br=O double bonds. Draw the Lewis structures of these possible resonance structures, and use formal charges to predict which makes the greatest contribution to the resonance hybrid.arrow_forwardWhat are the favored geometrical arrangements for ABn molecules for which the A atom has 2, 3, 4, 5, and 6 pairs of electrons in its valence shell?arrow_forwardA hypothetical covalent molecule, X–Y, has a dipole moment of 1.12D and a bond length of 101 pm. Calculate the partial charge on a pole of this molecule in terms of e, where e is the charge on an electron.arrow_forward
- Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol. (d) Using average bond enthalpies, estimate H for the formation of gaseous phosgene from CO(g) and Cl2(g).arrow_forwardDetermine the number of valence electrons in sulfuric acid (H₂SO₄) and then draw the corresponding Lewis structure (with minimized formal charges).arrow_forwardWrite the Lewis structure for nitric acid in which the three O atoms are bonded to the central N atom and the ionizable H atom is bonded to one of the O atom.arrow_forward
- If the dipole moment of a diatomic molecule is found to be 1.04 D, and its bond length is found to be 124 pm, what is the fractional charge on the atoms of the molecule in Coulombs (C)?arrow_forwardIf an element is bonded to 4 other atoms and has a formal charge of +1, what group must the element be in? I know that group 3A atoms are elctron deficient, and that period 3 elements and below, except for group 3A elements like Aluminum, can expand their octet because of their available d-orbital, which may not be relevant to this problem. I don't understand this question, or why the answer would be 5A. Is it because 5A have odd valence electrons, and can form free radicals, like NO?arrow_forwardDraw the Lewis dot structure and calculate the formal charges of the oxygen and phosphorus atoms of PO4 3- . What is the molecular geometry of the molecule?arrow_forward
- Draw the Lewis structure for the acids H2SO4 and H3PO4—note that acidic hydrogens for oxyacids are always attached to one of the oxygen atoms, and the central atom will break the octet rule in order to have zero formal charges.arrow_forwardWhich of the Lewis structures for NO is dominant based on analysis of the formalcharges?arrow_forwardDraw Lewis structures for these ions and show which atom in each bears the formal charge. Q.) H3O+arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


