
Concept explainers
The feed to a distillation column (sketched below) is a 45.0 mole% n-pentane−55.0 mole% n hexane liquid mixture. The vapor stream leaving the top of the column, which contains 98.0 mole% pentane and the balance hexane, goes to a total condenser (which means all the vapor is condensed). Half of the liquid condensate is returned to the top of the column as re?ux and the rest is withdrawn as overhead product (distillate) at a rate of 85.0 kmol/h. The distillate contains 95.0% of the pentane fed to the column. The liquid stream leaving the bottom of the column goes to a reboiler. Part of the stream is vaporized; the vapor is returned to the bottom of the column as boilup, and the residual liquid is withdrawn as bottom: product.
(a) Calculate the molar ?ow rate of the feed stream and the molar ?ow rate and composition of the bottoms product stream.
(b) Estimate the temperature of the vapor entering the condenser, assuming that it is saturated (at its dew point) at an absolute pressure of 1 atm and that Raoult's law applies to both pentane and hexane. Then estimate the volumetric ?ow rates of the vapor stream leaving the column and of the liquid distillate product. State any assumptions you make.
(c) Estimate the temperature of the reboiler and the composition of the vapor boilup, again assuming operation at 1 atm.
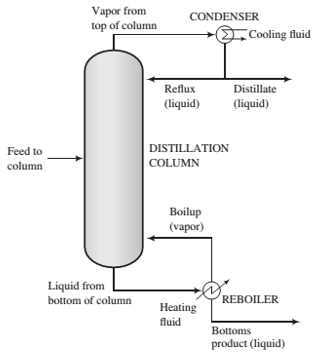
(d) Calculate the minimum diameter of the pipe connecting the column and the condenser if the maximum allowable vapor velocity in the pipe is 10 m/s. Then list all the assumptions underlying the calculation of that number.
Learn your wayIncludes step-by-step video

Chapter 6 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Experiencing MIS
Database Concepts (8th Edition)
Engineering Mechanics: Statics & Dynamics (14th Edition)
Introductory Circuit Analysis (13th Edition)
- 4.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint. The datd of four trials was collected. Please answer the following questions 1) calculate the total volume and moles of NaOH required to reach the endpoint for each trial. 2) calculate the molar solubility of potassium hydrogen tartate ( in mol/L) for each trial. 3) calculate the average molar solubility of potassium hydrogen tartate for the four trials. What is the average solubility of potassium hydrogen tartrate in g/L? 4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr…arrow_forwardUse only the first decimal points (X.X) for atomic masses. R = 8.314 L*kPa/mole*K. In a reactor at 350.0 kPA and 95 °C, HCl adds to acetylene by the following reaction:2HCl(g) + C2H2(g) --> C2Cl2H4(g)39.1 g of HCl and 39.1 g of acetylene are placed into the reactor. What is the limiting reagent? What is the final volume of the system?arrow_forwardA student determined the molar mass of an unknown ionic solid by the method used in this experiment. She found the equilibrium temperature of the ice water mixture (THE FREEZING POINT) to be 0.50°C on her thermometer. When she added 3.10 g of her solid to the mixture, the temperature fell to -4.30°C. She then poured off the solution into a beaker. The mass of the solution was 34.59 g. Kf = 1.86°C/m What was the freezing point depression ∆Tf (celsius) What was the molality of the unknown solution? How much water was in the solution? What did she find to be the observed molar mass of the ionic solid, assuming she made the calculation properly?arrow_forward
- 3. Refer to the phase diagram depicted below: When 7 moles of A and 3 moles of B are mixed at 50oC which is then cooled to 10oC the sample turns into two liquid phases, phase I has mole fraction xB=0.14 (see diagram below). Find the phase II mole fraction xB, and then calculate the ratio of amount of substance in phase I to that in phase II using Lever rule. Please enter your answer as a number with two decimals. for example, if the ratio you calculated is 2:3, you enter as 0.67arrow_forwardA 25mL volumetric pipet is used to deliver a sample of the stock solution marked “0.6000M X2SO4” into a 100mL volumetric flask. Distilled water is added to the flask until it is about 3/4thfull, the solution is mixed well, then more water is added to fill it up to the calibration mark, then it is mixed again. Calculate the concentration of X+ion in the dilute solution made above.arrow_forwardTwo process streams are mixed to form a single stream. Only the flow in the mixed stream is known. A soluble salt is added to one of the original streams at a steady rate. Samples taken of this stream show it to be 4.76 % w salt. Samples from the combined stream show 0.62% w salt. What is the ratio of the flows in the two original streams?arrow_forward
- 50.0 mL of 0.400 M NaOH (ag) was added to 20.0 mL of 0.500 M H2SO4 (ag) in a calorimeter of heat capacity 39.0 J/K. The temperature of the resulting solution rose by 3.60 K. Find AH° in kJ/mol for the neutralization of H2SO4 (ag) with NaOH (ag). Specific heat of aqueous mixture = 4.184 J/g.K. Density of mixture = 1.030 g/mL Carrow_forwardQ3/ A 100 mole feed containing equimolar amounts of methanol and water is mixe with 10 moles of a 40 mole% aqueous methanol stream. The mixture enters a distillation column that creates two streams. A top stream exits that contains 70 mole% methanol and balance with water. The bottom stream, which is 70 moles, enters a second distillation column. A top stream exits the second column as a 50% methanol and 50% water. The two top streams exiting the distillation column have the same flow rate. Calculate all unknown streams variables.arrow_forwardA tank contains 400 liters of brine holding 100kg of salt in solution. Water containing 125g of salt per liter flows into the tank at the rate of 12 liters per minute, and the mixture, kept uniform by stirring, flows out at the same rate. Find the amount of salt at the end of 90 minutes.arrow_forward
- (A) It is required to separate 1 mole of ethanol from ethanol-water mixture by using distillation Column at standard conditions. After 1 hour, it is found that the volume was double and the pressure increased to 152 kpa. Find the Temperature after 1 hour by using the ideal gas law: PV = nRT Given : R = 0.082 L.atm/mole.K (B) Calculate the capacity of a Base added to a solution contains 10 mole of ammonia (k, = 1.8x105) and 6 mole of ammonium chloride in 120 ml? Note: Atomic weight: H = 1, 0 = 16, C = 12, Ba = 137, Cl= 35 , N=14arrow_forwardvolume reactants gas products Na₂CO3 (s) + 2 HCI (aq) solution mass separate layer gram liquid solid coefficient CO₂ (g) + H₂O (1) + 2 NaCl (aq)|arrow_forwardA 100-gal tank initially contains pure water. A solution of dye containing 0.3 lb/gal flows into the tank at the rate of 5 gal/min and the resulting mixture flows out at the same rate. After 15 min. the process is stopped and fresh water flows into the tank at the rate of 5 gpm and the mixture flows out at the same rate. Find the concentration of dye in the tank at the end of 30 min.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





