
A dilute aqueous solution of H2SO4 (Solution A) is to be mixed with a solution containing 90.0 wt% H2SO4 (Solution B) to produce a 75.0 wt% solution (Solution C).
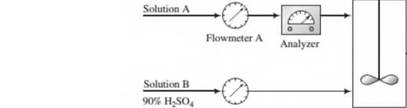
The flow rate and concentration of Solution A change periodically, so that it is necessary to adjust the flow rate of Solution B to keep the product H2SO4 concentration constant.
Flowmeters A and B have linear calibration plots of mass flow rate (m) versus meter reading (/?),
| which pass through the following points: | ||
| Flowmeter A: | mA = 150 lbn,/h, wa = 500 lbm/h. | «a = 25 «a = 70 |
| Flowmeter B: | riiB = 200 lbm/h, mB= 800 lbm/h. | Rb = 20 /?B = 60 |
The analyzer calibration is a straight line on a semilog plot of %H2SO4(x) on a logarithmic scale versus meter reading (/?,) on a linear scale. The line passes through the points (x = 20%, Rx= 4.0) and (x = 100%./?, = 10.0).
- Calculate the flow' rate of Solution B needed to process 300 lbm/h of 55% H2SO4 (Solution A), and the resulting flow rate of Solution C. (The calibration data are not needed for this part.)
- Derive the calibration equations for /ha(#a). «ib(^b), andx(/?v). Calculate the values of R\, Ru, and Rxcorresponding to the flow rates and concentrations of Part (a).
- The process technician's job is to read Flowmeter A and the analyzer periodically, and then to adjust the flow rate of Solution B to its required value. Derive a formula that the technician can use for Rbin terms of RAand Rx, and then check it by substituting the values of Part (a).
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Concepts Of Programming Languages
Management Information Systems: Managing The Digital Firm (16th Edition)
Heating Ventilating and Air Conditioning: Analysis and Design
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
- Two salts A and B are dissolved in water. At the temperature of the experiment, the solubility of A is 1 kg A/kg pure water and that of B is 0.4 kg /kg water. It will be assumed that the solubilities of the two salts are not affected by the presence of each other. If originally 20 kg of A and 20 kg B are dissolved in 100 kg water, and some of the water is evaporated from solution, calculate the: (1) amount evaporated (2) weight of mother liquor (3) composition of mother liquor (4) weight of each crystal formed for the following cases: a) 50% of the water is evaporated b) Enough water is evaporated to reduce the total weight of the solution to 50% of the original value c) Enough water is evaporated to leave the solution saturated with A, without crystallizing any A d) Enough water is evaporated so that the remaining solution is 50% of the original value.arrow_forwardA variation of the indicator-dilution method (see preceding problem) is used to measure total blood volume. A known amount of a tracer is injected into the bloodstream and disperses uniformly throughout the circulatory system. A blood sample is then withdrawn, the tracer concentration in the sample is measured, and the measured concentration [which equals (tracer injected)/(total blood volume) if no tracer is lost through blood vessel walls] is used to determine the total blood volume.In one such experiment, 0.60 cm3 of a solution containing 5.00 mg/L of a dye is injected into an artery of a grown man. About 10 minutes later, after the tracer has had time to distribute itself uniformly throughout the bloodstream, a blood sample is withdrawn and placed in the sample chamber of a spectrophotometer. A beam of light passes through the chamber, and the spectrophotometer measures the intensity of the transmitted beam and displays the value of the solution absorbance (a quantity that…arrow_forwardFor the purpose of determination of agricultural pesticides in river water, 3.2 liters of river water were taken and extracted with 125ml of CCI4 solvent using a separating funnel. After shaking and reaching the state of equilibrium, if you know that the value of the distribution coefficient (KD) is equal to 20, the weight ratio of the pesticides in the boiling layer to the lower layer in the separating funnel?arrow_forward
- b) A gaseous mixture contain 32.9 mol % He, 40.7 mol % N2 and 26.4 mol % Ar. Determine the composition of this mixture on a mass basis based on the molecular weight given: MW (He) = 4.003 , MW (N2) = 28.02, MW(Ar) = 39.95 %3Darrow_forwardTwo aqueous sulfuric acid solutions are available at a processing facility: 20.0 wt% H2SO4 (sg = 1.139) 60.0 wt% H2SO4 (sg = 1.498) It is desired to mix the two solutions such that the final product has a concentration of 4.00 M (sg-1.213). There is 100 kg of the 20% solution available. If all of this is to be used, calculate: The mass of 60% solution needed The mass and composition of the final productarrow_forwardTetracycline produced in Streptomyces aureus fermentations is purified by crystallisation. One hundred kg of a supersaturated solution containing 7.7 wt% tetracycline is cooled in a batch fluidised-bed crystalliser. Seed crystals of tetracycline are added at a concentration of 40 ppm to promote crystal growth. At the end of the crystallisation process, the remaining solution contains 2.8% tetracycline. (a) What is the mass of the residual tetracycline solution? (b) What mass of tetracycline crystals is produced?arrow_forward
- Calculate the solubility at 25 °C of PbCO3 in pure water and in a 0.0100M Pb(NO3), solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: 0² solubility in 0.0100 M Pb(NO3)2 g 0- solution: 6.0 6.0 ☐ x10 X 09 ☐☐ S 00 ?arrow_forwardHow to prepare the solutions listed below Solution 1) 25 mL 7 M sulfuric acid and the respective dilution of this solution to prepare 50 mL 2 M sulfuric acid in deionised water. Sulfuric acid Mw = 98.079 g mol-1 Sulfuric acid density = 1.84 g mL-1 Sulfuric acid purity = 98% Solution 2) 100 mL 0.02M potassium permanganate potassium permanganate Mw = 158.03 g mol-1 potassium permanganate purity = 99%arrow_forwardHydroxyapatite, Ca₁0 (PO4) 6 (OH)2, has a solubility constant of Ksp = 2.34 x 10-59, and dissociates according to Ca10 (PO4)(OH)2(s) = 10 Ca²+(aq) + 6 PO¾-¯(aq) + 2 OH(aq) Solid hydroxyapatite is dissolved in water to form a saturated solution. What is the concentration of Ca²+ in this solution if [OH] is fixed at 3.60 × 10-4 M? [Ca2+] = Marrow_forward
- A steady-state process to recover crystalline potassium chromate (K,CrOz) from an aqueous solution of this salt is required. Four thousand kilograms per hour of a solution that is one-third K,CrO, by mass is joined by a recycle stream containing 36.4% K2CrO7, and the combined stream is fed into an evaporator. The concentrated stream leaving the evaporator contains 49.4% K,CrO; this stream is fed into a crystallizer in which it is cooled (causing crystals of K,CrO, to come out of solution) and then filtered. The filter cake consists of K,CrO, crystals and a solution that contains 36.4% K,CrO, by mass; the crystals account for 95% of the total mass of the filter cake. The solution that passes through the filter, also 36.4% K,CrO,, is the recycle stream. 1- Draw the flowchart of the system and put all known information. 2- Calculate the rate of evaporation, the rate of production of crystalline K,CrO7, the feed rates that the evaporator and the crystallizer must be designed to handle, and…arrow_forward令 。 B/s 因 1:Y9 404 2_543841247.. Use of the Normal Law Normality (N) = Wt /Eq.wt 1000/V(ml) Wt (gm) =Weight of sodium carbonate V (ml) = solution volume N= Normality Discussion: 1. What is the equivalent weight of the vehicle? primary standard? 2. Why is sodium carbonate 3- Attend naciN 3.3 solution in a 253 mi volume bottle. 4- Calculate the caliber of sodium carbonate if the weight of sodium carbonate gm 2 and the size of the bottle volume is 100ml Results Sheet Name: Group: Date: Signature 1-Calculations: 2-Aim of experiment: 3-Theoretical results M(Na2Co3).. .. Weight required= 4-Observations: 个arrow_forwardA 3 m^3 drum contains a mixture at 101 KPa and 35 C of 60% Methane (CH4) and 40% oxygen (O2) on a volumetric basis. a. Determine the amount of oxygen that must be added at 35 C to change the volumetric analysis to 50% of each component.arrow_forward
