
In the production of a bean oil, beans containing 13.0 wt% oil and 87.0% sobds are ground and fed to a stirred tank (the extractor ) along with a recycled stream of liquid /i-hexane. The feed ratio is 3 kg hexane/kg beans. The ground beans are suspended in the liquid, and essentially all of the oil in the beans is extracted into the hexane. The extractor effluent passes to a filter where the solids are collected and form a filter cake. The filter cake contains 75.0 wt% bean solids and the balance bean oil and hexane, the latter two in the same ratio in which they emerge from the extractor. The filter cake is discarded and the liquid filtrate is fed to a heated evaporator in which the hexane is vaporized and the oil remains as a liquid. The oil is stored in drums and shipped. The hexane vapor is subsequently cooled and condensed, and the liquid hexane condensate is recycled to the extractor.
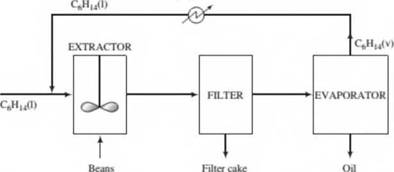
- Draw and label a flowchart of the process, do the degree-of-freedom analysis, and write in an efficient order the equations you would solve to determine all unknown stream variables, circling the variables for which you would solve.
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Electrical Engineering: Principles & Applications (7th Edition)
Using MIS (10th Edition)
Thinking Like an Engineer: An Active Learning Approach (3rd Edition)
Starting Out with Python (4th Edition)
- Q4/in a two stage process, acetic acid (A)is extracted from water (W) into hexanol (H) in a liquid-liquid extraction vessel and the extract is subsequently separated by distillation. Assume that water is completely insoluble in hexanol. A mixture of 18wt% acetic acid and the balance water is feed to liquid-liquid extraction vessel. Pure hexanol is feed to the column to extracted the acetic acid. The water-rich stream leaving the vessel is 99.5wt% water and the balance acetic acid. The hexanol-rich extract from the vessel is feed to a distillation column. The composition of the distillate is 96wt% acetic acid and the balance hexanol. The bottom stream contains 97.2wt% hexanol and recovers 95% of the hexanol feed to the liquid-liquid extraction vessel. Calculate the percentage of acetic acid in the process feed that is recovered in the distillate stream. Distillation Liq-liq Column Extractionarrow_forwardFeed gas containing of 78.5mol % H₂, 21% of N₂ & 0.5% of Ar is mixed with recycle gas and enters a reactor where 15% N₂ is converted to NH3 as per the reaction. Ammonia from the exit of the reactor is completely separated from unconverted gases. To avoid the buildup of inerts, a small fraction (5%) of the unreacted gases purged and the balance recycled. USING ASPEN/HYSYS Draw the process flow sheet Product rate and Purge rate Basis:100mol/hrarrow_forwardIn a process producing KNO3 salt, 1000 kg/h of a feed solution containing 10 wt% KNO3 is fed to an evaporator, which evaporates some water at 422 K to produce a 50 wt% KNO3 solution. This is then fed to a crystallizer at 311 K, where crystals containing 98 wt% KNO3 are removed. The saturated solution containing 37.5 wt% KNO3 is recycled to the evaporator. Calculate the amount of the recycle stream R in kg/h and the product stream of crystals P in kg/h.arrow_forward
- Solid calcium (CaF2) reacts with sulfuric acid to form solid calcium sulfate and gaseous hydrogen fluoride. The HF is then dissolved in water to form hydrofluoric acid. A source of calcium fluoride is fluorite ore containing 96 wt% CaF2 and 4% SiO2. In a typical hydrofluoric acid manufacturing process, fluorite ore is reacted with 93 wt% aqueous sulfuric acid, supplied 15% in excess of the stoichiometric amount. Ninety-five percent of the ore dissolves in the acid. Some of the HF formed reacts with the dissolved silica in the reaction 6 HF + SIO2 (aq) – H2SİF6 (9) + 2 H2O (1) The hydrogen fluoride exiting from the reactor is subsequently dissolved in enough water to produce 60 wt% hydrofluoric acid. Calculate the quantity of fluorite ore needed to produce a metric ton of acid. Express your answer into three significant figures.arrow_forward2. In an ethanol production plant, a separator produces a 99% ethanol product from a feedstock stream containing 85% water and 15% ethanol at a rate of 450 Ib/min. The separator has two outlet streams: the ethanol product outlet stream (99% ethanol) and a residual water stream. 30% of the feedstock is bypassed and mixed with the residual water stream leaving the separator. If 60% of the ethanol entering the separator is recovered in the product stream, determine the composition of the residual water stream just after leaving the separator and the composition of the residual stream after mixing with the bypass stream.arrow_forwardA steady-state process to recover crystalline potassium chromate (K,CrOz) from an aqueous solution of this salt is required. Four thousand kilograms per hour of a solution that is one-third K,CrO, by mass is joined by a recycle stream containing 36.4% K2CrO7, and the combined stream is fed into an evaporator. The concentrated stream leaving the evaporator contains 49.4% K,CrO; this stream is fed into a crystallizer in which it is cooled (causing crystals of K,CrO, to come out of solution) and then filtered. The filter cake consists of K,CrO, crystals and a solution that contains 36.4% K,CrO, by mass; the crystals account for 95% of the total mass of the filter cake. The solution that passes through the filter, also 36.4% K,CrO,, is the recycle stream. 1- Draw the flowchart of the system and put all known information. 2- Calculate the rate of evaporation, the rate of production of crystalline K,CrO7, the feed rates that the evaporator and the crystallizer must be designed to handle, and…arrow_forward
- In a chemical production plant, cyclohexane is made by the reaction of benzene and hydrogen. Reaction is as follows: CHs + H2 → CoH12 The complete process of producing cyclohexane uses a reactor and a separator. Using the process shown below and assuming 20 % excess hydrogen in the fresh feed, find the ratio of the recycle stream to the fresh feed stream, the composition of the final product stream, and the composition of the stream leaving the reactor. The overall conversion of benzene is 92% and the single-pass conversion is 21.5 %. The recycle stream is composed of 23.9 % benzene and the remaining hydrogen. Fresh Feed REACTOR SEPARATOR Product Recyclearrow_forwardQ3/ A 100 mole feed containing equimolar amounts of methanol and water is mixe with 10 moles of a 40 mole% aqueous methanol stream. The mixture enters a distillation column that creates two streams. A top stream exits that contains 70 mole% methanol and balance with water. The bottom stream, which is 70 moles, enters a second distillation column. A top stream exits the second column as a 50% methanol and 50% water. The two top streams exiting the distillation column have the same flow rate. Calculate all unknown streams variables.arrow_forwardA hydrocarbon stream composed of 40 mol% n-butane and 60 mol% n-octane is fed at 100 kmol/h to a flash vessel operating at 5 bar and 145°C. The liquid outlet stream of the flash vessel is then sent to a distillation column with a partial condenser to further separate the components. 90% of the butane in the feed to the column is recovered in the overhead vapor product (distillate), along with small amounts of octane. The bottoms product contains octane which accounts for 75% of the octane in the fresh hydrocarbon feed (to the flash vessel). The condenser and reboiler operating temperatures are 136.7°C and 165.7°C, respectively. Determine the compositions of the liquid outlet stream of the condenser and the vapor outlet stream of the reboiler.arrow_forward
- Two aqueous sulfuric acid solutions are available at a processing facility: 20.0 wt% H2SO4 (sg = 1.139) 60.0 wt% H2SO4 (sg = 1.498) It is desired to mix the two solutions such that the final product has a concentration of 4.00 M (sg-1.213). There is 100 kg of the 20% solution available. If all of this is to be used, calculate: The mass of 60% solution needed The mass and composition of the final productarrow_forwardSea water containing 4 wt. % salt (NaCI) is passed through a reverse osmosis (RO) membrane filtration unit to produce a "fresh" water product containing 0.5 wt.6 NaCI and a concentrated brine waste stream. If there are 28 kg of fresh water recovered from every 100 kg of sea water, then determine the concentration of the brine waste stream. Type your answer in as a weight percentage rounded off to one decimal place, but do not include the 6 symbol.arrow_forward3. Given: Soda and lime are added to a glass batch in the form of soda ash (Na,CO,) and limestone (CaCO). During heating, these two ingredients decompose to give off carbon dioxide (CO₂), the resulting products being soda and lime. Find: Compute the weight of soda ash and limestone that must be added to 110 lb of quartz (SiO₂) to yield a glass of composition 72 wt% SiO₂, 16 wt% Na₂O, and 12 wt% Cao. Solution:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





