
A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a sulfur recover)' reactor, where the reaction
2H2S + SO2 -» 3S + 2H2O
takes place. The feed rates are adjusted so that the ratio of H2S to SO2 in the combined feed is always stoichiometric.
In the normal operation of the reactor the flow rate and composition of the H2S feed stream both fluctuate. In the past, each time either variable changed the required SO2 feed rate had to be reset by adjusting a valve in the feed line. A control system has been installed to automate this process. The H2S feed stream passes through an electronic flowmeter that transmits a signal Rf directly proportional to the molar flow rate of the stream, h{. When = 100 kmol/h, the transmitted signal R(= 15 mV. The mole fraction of H2S in this stream is measured with a thermal conductivity detector, which transmits a signal /fa. Analyzer calibration data are as follows:
| fla(mV) | 0 | 25.4 | 42.8 | 58.0 | 71.9 | 85.1 |
| x(mol H2S/mol) | 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
The controller takes as input the transmitted values of Rf and R3and calculates and transmits a voltage signal j?c to a flow control valve in the SO2 line, which opens and closes to an extent dependent on the value of Rc. A plot of the SO2 flow rate. fic, versus Rcon rectangular coordinates is a straight line through the points (Rc= 10.0mV.hc= 25.0kmol/h) and (/<. = 25.0mV.hc = 60.0kmol/h).
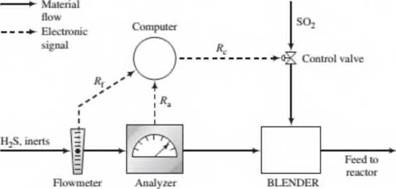
- Why would it be important to feed the reactants in stoichiometric proportion? (Hint: SO2 and especially H2S are serious pollutants.) What are several likely reasons for wanting to automate the SO2 feed rate adjustment?
- If the first stream contains 85.0 mole% H2S and enters the unit at a rate of hf = 3.00 X 102 kmol/h, what must the value of nt(kmol SO2/h) be?
- Fit a function to the H2S analyzer calibration data to derive an expression for x as a function of Rt. Check the fit by plotting both the function and the calibration data on the same graph.
- Derive a formula for Rcfrom specified values of Rf and Ra, using the result of Part (c) in the derivation. (This formula would be built into the controller.) Test the formula using the flow rate and composition data of Part (a).
- The system has been installed and made operational, and at some point the concentration of H2S in the feed stream suddenly changes. A sample of the blended gas is collected and analyzed a short time later and the mole ratio of H2S to SO2 is not the required 2:1. List as many possible reasons as you can think of for this apparent failure of the control system.
Trending nowThis is a popular solution!

Chapter 4 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Applied Fluid Mechanics (7th Edition)
Starting Out With Visual Basic (7th Edition)
Artificial Intelligence: A Modern Approach
Starting Out with Python (3rd Edition)
- .edu bryuitra/courses roee_re Question Completion Status: The following process describes the complete combustion reaction of Toluene fuel C7HB (SG 0.866) with air. Liquid fuel (n1) enters the combustion chamber at 200mL/min, where it evaporated into a stream of air (n2) 15% in excess. Complete combustion reaction in which only fraction of the fuel is burned. The combustion products go to a condenser, where the unreacted fuel and water are liquified. The mass flowrate of the fuel in this steam (ma) is 50. The uncondensed gas leaves the condenser at 60 C and 0.5 atm gauge pressure. Calculate the fractional conversion of the fuel Calculate the SCMH of the air entering the combustion chamber Calculate the volumetric flow rate of the gas leaving the condenser (m³/min). a. b. C. ri4 CO2 n3 Fuel ris N2 Fuel ri1 ni4 CO2 nig O2 Combustion nig N2 Condenser air riz Chamber ng O2 ni, H,0 n3 Fuel niz H,0arrow_forwardPropane is dehydrogenated to from propylene in a catalytic reactor: C3H8 → C3H6 + H2 The process is to be designed for a 95% overall conversion of propane. The reaction products are separated into two streams: the first, which contains H2, C3H6, and 0.555% of the propane that leaves the reactor, is taken off as product; the second stream, which contains the balance of the unreacted propane and 5% of the propylene in the product stream, is recycled to the reactor. Calculate the composition of the product, the ratio (moles recycled)/(moles fresh feed), and the single-pass conversion.arrow_forwardAn exothermic reaction is taking place in a constant volume reactor that contains a cooling coil. The reaction A -> B proceeds with a reaction rate constant of k1. The rate of reaction is given as kCA. The feed concentration of A is 4 mol/L, the reaction conversion is 50%, and the reaction rate constant k is 1*10-2 h^-1. The volumetric flow rate remains constant throughout the reaction, at 2 L/hr. The initial temperature in the reactor is 40°C, and the final temperature is 60°C. The heat released at the end of the reaction is 60 j/mol. The amount of heat removed by the coil is 400 j. The density of the mixture is 4 kg/m^3, the Cp value is 20 j/mol.K. If the reactor volume is 4 liters, find the change in temperature over time.arrow_forward
- Consider the following simultaneous reactions: N2 + 3H2 → 2NH3(a) H2 + CO2 → CO + H2O (b) 550 mols are fed into the reactor, initially containing 67.4% H2, 21.3% N2 and the remainder CO2. The mixture at the outlet has 16% NH3 and 5% H2O (molar percentages). a- Calculate the degrees of advancement of reactions (a) and (b).arrow_forward72.- The elementary reaction A + B C + D is carried out in a CSTR in which it is suspected there is bypass and dead volume. The table attached shows the step tracer concentrations at the output of the system. If the theoretical volume of the reactor is 1 m', the feeding volumetric flow rate is 0.1 m'/min, the rate constant is 0.28 m'/(kmol-min) and the feeding consists of an equimolar solution of A and B, with C = 2 kmol/m', calculate the conversion that can be expected in the system. %3D F=C t (min) 4 8. 10 14 16 18 00 C (mg/L) 1000 1333 1500 1666 1750 1800 2000 my Activate Windows Gto Settings to activalarrow_forwardReferring to a continuous stirred tank reactor (CSTR), determine the reactor volume required to process 1,200 L/min of 6 M reactant A in a reaction of A → B given a conversion of 90%. The reaction rate constant, k = 2.0 min-1.arrow_forward
- Extensions Help kt 10 Calibri 6 1 4 1.3 Y 2 f7 12 + BIUA 7. Scrubbers are a type of pollution control technology installed on industry smokestacks to capture sulfur dioxide before it is emitted to the atmosphere. Once collected, the sulfur dioxide can be used to manufacture sulfuric acid, which can be sold to other companies that make &7 U 3 1 10. Why is it essential to continually monitor air and water quality? detergents. Explain how the use of scrubber technology benefits both industry and the environment. 8 8. Most North American automobile manufacturers are shifting their production from gasoline powered vehicles to electric vehicles. What effect should this trend have on acid rain? 4 fg Explain how the concentration of a pollutant is related to the severity of its impact on the environment. Lesson 7: Remediate Chemical Pollution f10 5 hp insert 9 E ▼ EYE- f11 6 O f12 mismatisla THEOLOG prt scr 7 G BRUCK 63°F Cloudy delete backspace Earrow_forwardll T-Mobile Wi-Fi ? 2:31 PM @ 7 51% 23obrienm Fer... CHE222 Ch 9.2 Balancing & Classifying Chemical Reactions Types of Chemical Reactions Balancing Chemical Reactions 1)_ N, + H, - NH, 2) KCIO, → _ KCl + O, 3) NaCl + F, -__ NaF + Cl, 4) Н, + H̟0 5) _CH, + 0, →. СО, + H,O 6) _C,H, +_ 0, → __CO, + H,0 7) С,Н, + 0,- _CO, + H,0 8) P + _0, → __P,O, 9) Na + H,O → NaOH + H, 10) Ag,O → __Ag + _0, 11) S, + _SO, 12) K + MgBr,→ KBr + _Mgarrow_forwardTitanium (Ti) is an important material in manufacturing airplane engines and frames. It is obtained from titanium tetrachloride (TiCl4), prepared from titanium dioxide, carbon, and chlorine through the following reaction: 3TiO2(s) + 4C(s) +6C2(g) -------à 3TiC4(g) 2CO2(g) + 2CO(g) a. If the reactor contains 11.34 g C, 13.56 g Cl2, and 8.3 g TiO2, identify the limiting reagent. b. Compute the theoretical yield of TiCl4. After the actual experiment, the reaction produces 13.48 g TiCl4. What is the percent yield of TiCl4?arrow_forward
- Consider the calcination of CaCO3: CaCO3 -> CaQ + CO, Before calcination, the initial weight of CaCO3 is 2.5g. After calcination, the final weight of CaCO3 was measured at 2.0 g. Calculate the conversion of CaCO; particles (x) using the following equation: m CaCO, -m CaCo, ) x = m Caco, -MCao |arrow_forwardFigure below shows three reactors linked by pipes. As indicated, the rate of transfer of chemicals through each pipe is equal to a flow rate (Q, with units of cubic meters per second) multiplied by the concentration of the reactor from which the flow originates (c, with units of milligrams per cubic meter). If the system is at a steady-state, the transfer into each reactor will balance the transfer out. Develop mass-balance equations for the reactors and solve the three simultaneous linear algebraic equations for their concentrationsarrow_forwardThe following series of reactions were carried out. PbCO3(s) +2HNO3(aq) ---> Pb(NO3)2(aq) +H2O(l)+CO2(g) Pb(NO3)2(aq) + 2HCl(aq) ---> 2HNO3(aq) +PbCl(s) (a) if a student startes with 2.871 g of lead(II) carbonate for the first reaction and all other reagents are in excess, what is the theoretical yield of lead(II) chloride solid? (b) If the student isolates 2.385 g of lead(II) chloride, what is the percent yield. (please indicate which are the final answers, thank you.)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





