
Oxygen consumed by a living organism in aerobic reactions is used in adding mass to the organism and/or the production of chemicals and carbon dioxide. Since we may not know the molecular compositions of all species in such a reaction, it is common to define the ratio of moles of CO2produced per mole of O2 consumed as the respiratory quotient. RQ. where
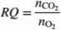
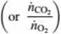
Since it generally is impossible to predict values of RQ, they must be determined from operating data. Mammalian cells are used in a bioreactor to convert glucose to glutamic acid by the reaction
CftHiiOf, + £/NHj + hO2 —? PC5H9NO4 + t/CO2 + rfl2O
The feed to the bioreactor comprises l.OOx 102 mol C6H12O(/day, 1.20x 102 mol NHj/day, and l.lOx 102 mol O2/day. Data on the system show that RQ = 0.45 mol CO2 produced/mol On consumed.
- Determine the five stoichiometric coefficients and the limiting reactant.
Trending nowThis is a popular solution!

Chapter 4 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Manufacturing Engineering & Technology
Starting Out with C++: Early Objects
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Applied Statics and Strength of Materials (6th Edition)
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 30.0 cm wide and 36.0 cm high. The maximum safe pressure inside the vessel has been measured to be 8.20 MPa. For a certain reaction the vessel may contain up to 2.32 kg of dinitrogen difluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: C x10 × Sarrow_forwardGiven the reaction: 2 B(s) + 3 H2(g) ↽−−⇀ 2 BH3(g) at 25 oC. At equilibrium, 0.254 moles BH3, 0.588 moles H2 and 0.666 mole B are found in a 1.0 L flask. Determine the value of Kc. Express the answer to 3 sig. figs.arrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 19.0 cm wide and 22.8 cm high. The maximum safe pressure inside the vessel has been measured to be 3.60 MPa. For a certain reaction the vessel may contain up to 0.346 kg of sulfur tetrafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits. temperature:|°Carrow_forward
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 30.0 cm wide and 36.0 cm high. The maximum safe pressure inside the vessel has been measured to be 1.80 MPa. For a certain reaction the vessel may contain up to 0.834 kg of sulfur tetrafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: |C x10, Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility image_50363137.JPGarrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 13.0cm wide and 15.6cm high. The maximum safe pressure inside the vessel has been measured to be 3.60MPa. For a certain reaction the vessel may contain up to 0.0457kg of carbon dioxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits.arrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 60.0cm wide and 72.0cm high. The maximum safe pressure inside the vessel has been measured to be 2.60MPa.For a certain reaction the vessel may contain up to 4.86kg of boron trifluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digitsarrow_forward
- Consider the following reaction where Ke = 9.52x10 at 350 K. CHa(9) + CCla(0) =2CH,Cl2(9) A reaction mixture was found to contain 3.07x102 moles of CHa(g), 3.96x10 moles of CCIA(g) and 1.21x102 moles of CH,Cl2(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qo equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forwardIf 8g of ammonium hydroxide is completely decomposed by the reaction below in a closed container with a total volume of 5.00L and at 25°C, what will be the partial pressure of the ammonia gas liberated, in atmospheres? Assume the volume of the liquid is negligible. NH4OH(aq) --> H2O(l)+NH3(g)arrow_forwardConsider the following reaction CS2(g) + 3O2(g) → CO2(g) + 2SO2(g) A mixture containing only CS2(g) and excess O2(g) at a total pressure of 100 kPa is placed in a sealed vessel. After the reaction is completed and the vessel is cooled to the initial temperature, the total pressure in the vessel drops to 80 kPa. What is the mole fraction of CO2(g) in the final mixture?arrow_forward
- Underwater, the pressure increases by 1 bar for every 10 m of depth. When a scuba diver breathes pressurized air in which the partial pressure of N2(g) exceeds 4 bar, the result is nitrogen narcosis, which is similar to alcohol intoxication. What is the maximum mole percent of N2 in an air mixture that could be used by divers at depths up to 85 m below sea level without undue risk of nitrogen narcosis?arrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 43.0 cm wide and 51.6 cm high. The maximum safe pressure inside the vessel has been measured to be 8.30 MPa. For a certain reaction the vessel may contain up to 15.7 kg of chlorine pentafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits. temperature: °C ☐ 10 Xarrow_forward5. Gases: You analyze that principal components of a gas inside a valve. Through spectroscopy, you were able to analyze that components of the gas mixture are 02, N2 and H2 respectively. From that you would like to check if you have learned something from GCHEM203A by determining (a) what are the mole fractions of each component in the mixture of 15.08 g of O2, 8.17 g of N2, and 2.64 g of H2? (b) What is the partial pressure in atm of each component of this mixture if it is held in a 15.50-L vessel at 15 °C?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
