
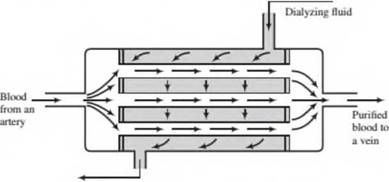
An artificial kidney is a device that removes water and waste metabolites from blood. In one such device, the hollow fiber hemodialyzer, blood flow's from an artery through the insides of a bundle
of hollow' cellulose acetate fibers, and dialyzing fluid, which consists of water and various dissolved salts, flows on the outside of the fibers. Water and waste metabolites—principally urea, creatinine, uric acid, and phosphate ions—pass through the fiber walls into the dialyzing fluid, and the purified blood is returned to a vein.
Dialysate
At some time during a dialysis the arterial and venous blood conditions are as follows:
Arterial (entering) Blood Venous (exiting) Blood
| Flow Rate | 200.0 mL/min | 195.0 mL/min |
| Urea (H2NCONH2) Concentration | 1.90 mg/mL | 1.75 mg/mL |
- Calculate the rates at w hich urea and water are being removed from the blood.
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Experiencing MIS
Modern Database Management (12th Edition)
Absolute Java (6th Edition)
Fundamentals of Heat and Mass Transfer
- A water containing 50 mg/L of phosphate (PO,") is flowing in an open channel at a volumetric flow rate (Q) of 2 MGD (million gallons per day). (a) What is the concentration of phosphate expressed in (i) mg/L as PO, --P, (ii) mM as PO., and (iii) mM as PO, P. (b) Find the mass flow rate (S) of phosphate through any section perpendicular to the direction of flow in the open channel expressed in lb/day? Given: Atomic weights: P= 31 g/mole, O = 16 g/mole, 1 gallon = 3.785 L, 1 kg = 2.204 Ib. %3Darrow_forwardYou carry out an experiment on the physical properties of 3 organic compounds and observe the following data: Substance Solubility in Water medium Compound A low Compound B high Compound C You know that the 3 organic compounds are an alkene, ketone and alcohol of equal hydrocarbon length. Match the compounds above to their specific organic compound type based upon their solubility. And give a structural reason for the solubility observations that are observed. (I:3) Substance Identity Reason Compound A Compound B Compound Carrow_forwardA 10 g sample of Mg504 · 7H/₂0 (246.48 2/mp) oven at 105 °C was heated in an until all the waters of hydration was evaporated off to generate MgSO4 (mhydrous). The sample was then dissolved in 250 mL of distilled water and diluted to a final volume of amount of 504²- in ppm in 0.56. The the final solution is ? motor masses in g/mol = My5o4 · 71/₂0 7/₂0 = 206 246.48, H/₂0 = 18.02 طلاarrow_forward
- Mganga is doing a routine analysis in a copper plating factory. He is regularly making up solutions of verypure CuSO4.5H2O. He suspect that a batch of a reagent used to make up a stock solution of CuSO4.5H2Ois impure and it may contains some CuCl2. He asked you to determine the percentage impurity as follows:Dissolve1.500g of a mixture and make up to 1.00L mark.150mL of this solution was treated with 20.00mLof 0.100M AgNO3 to remove 98.6% of chloride as AgCl precipitate.arrow_forwardWhen pure furfural (solvent) is added to a mixture containing 0.2 mass fraction diphenylhexane and 0.8 mass fraction docosane, two separate layers can be obtained. If the composition of the two layers are as shown in the table, how much solvent was added to 500 kg of solution of docosane and diphenylhexane?arrow_forwardA spent 1.0 percent caustic soda solution (ρNaOH = 8.42 lb/gal) with a volumetric flow rate of 3.0gpm is to be neutralized using either sulfuric acid or hydrochloric acid. First, determine the massflow rate (lb/day) of NaOH in the spent caustic soda solution, and then use that value todetermine the stoichiometric amounts (lb/day) of:1. H2SO4 required for neutralizing the OH− ions contributed by the spent caustic soda.[Hints: (1) write the balanced chemical equation for the acid-base reaction betweenNaOH and H2SO4, and use it to establish the stoichiometric weight ratio H2SO4 : NaOH.]2. HCl required for neutralizing the OH− ions contributed by the spent caustic soda. [Hints:(1) write the balanced chemical equation for the acid-base reaction between NaOH andHCl and use it to establish the stoichiometric weight ratio HCl : NaOH.]arrow_forward
- One technique for studying the energetics of protein structure is to monitor the struc- ture of the protein as a function of the osmotic pressure. In these experiments the os- motic pressure of the solution is varied by using polymers of different molecular weight and concentration. In an experiment poly(ethylene glycol) (PEG) with a molecular weight of 1000.0 g mol- was used. What concentration of PEG is needed to create an aqueous solution with an osmotic pressure of 150.0 Pa at 25°C? You may assume that PEG is uncharged and that it does not dissociate or aggregate.arrow_forwardA sodium sulfate solution is measured at 2.2 molal in pure water at a source company. (sodium sulfate molar mass = 142.05 g mol) The lab needs to report the concentration in molarity. What is the final molarity of the sodium sulfate solution? (density of water = 1.0 g mL¯' ; density of the solution = 1.2 g uestion 4 %3D mL-1). (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) Question 4 of 29> AMoving to another question will save this response. 888. P11 64 F12 eIC " 24 4 & 7\ 3 5 6. 8. %3D dele E R T Y A S D L F G K Z C N alt alt option command command optionarrow_forwardSea water containing 4 wt. % salt (NaCI) is passed through a reverse osmosis (RO) membrane filtration unit to produce a "fresh" water product containing 0.5 wt.6 NaCI and a concentrated brine waste stream. If there are 28 kg of fresh water recovered from every 100 kg of sea water, then determine the concentration of the brine waste stream. Type your answer in as a weight percentage rounded off to one decimal place, but do not include the 6 symbol.arrow_forward
- iverity at Anbar Cellege for Pure Belenees Analytical Chemistry 1// 1 Stage Dr. Bashar AbdulazeeR Mahmood Peperiment of Chemistry H.W: date the Formal and Molar concentrations of the constituents in 2.30 g of ethanol (g. fw%3D ) in 3.50 litres of aqueous solution. 4) Molal concentration (Molality) m The solution concentration produce from dissolved solute (mole) in solvent (kg), molality oes not change with temperature and used for physicochemical measurements. Example: Calculate the molal concentration for solution preparing from mixing 4 g NAOH with 500 g water. W 1000 4 g 1000 Solution: Molality (m) = 0.2 m %3D M.w w (g) 40 g/mol 500 (5) Concentration by percent Chemists frequently express concentrations in term of percentage Common methods include: a. Weight percent (w/w): It is the number of grams of solute per g of solvent or solution (w/w) E-) w of solute (g) w of solution or sample (g) w of solute (mg) w of solution or sample (mg) -%- %3D x 100 = %3D X 1arrow_forward4. In the water and glycerol mixture, the relationship between molar volume and mol fraction of glycerol is given in the following table at 20°C: X₂ (mol fraction of glycerol) 0 Molar volume (cm³ mol-¹) 18.05 19.18 20.53 24.18 0.0212 0.0466 0.1153 0.2269 0.4390 55.87 0.6923 1 73.02 (a) Calculate the partial molar volume of water and glycerol as a function of X2. (b) Show that the molar volume in the table can be calculated using the partial molar volume. 30.21 41.82arrow_forwardA water carbonating plant is available for use in the home and operates by providingcarbon dioxide at 5.0 atm. Estimate the molarity of the soda water it produces. Hint:Henry’s Law can also be expressed as PB = mBKB where PB is the partial pressure ofsubstance B, mB is the molality of the solution, and KB would be the Henry’s Lawconstant of B. Using this expression, the Henry’s Law constant for CO2 is 3010kPa*kg/mol. You may assume that the density of water is approximately 1.0 g/mL. A) 0.17 MB) 0.017 MC) 0.067 MD) 0.0017 ME) Cannot be determinedarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





