
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.6, Problem 1.4ACP
Use the atomic radii of scandium, yttrium, lanthanum, and lutetium to answer the questions below.
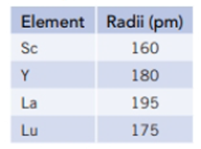
- a. Explain why lutetium has a smaller atomic radius than lanthanum, even though it has a greater number of electrons.
- b. Do the atomic radii argue for the placement of La or Lu below Y in the periodic table? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 7 Solutions
Chemistry & Chemical Reactivity
Ch. 7.3 - (a) What element has the configuration...Ch. 7.3 - Write one possible set of quantum numbers for the...Ch. 7.3 - Using the periodic table and without looking at...Ch. 7.4 - Prob. 7.4CYUCh. 7.5 - Without looking at the figures for the periodic...Ch. 7.6 - The most common oxidation state of a rare earth...Ch. 7.6 - Prob. 1.2ACPCh. 7.6 - Prob. 1.3ACPCh. 7.6 - Use the atomic radii of scandium, yttrium,...Ch. 7.6 - Prob. 1.5ACP
Ch. 7.6 - Prob. 1.6ACPCh. 7.6 - Give the electron configurations for iron and the...Ch. 7.6 - Prob. 2.2ACPCh. 7.6 - Prob. 2.3ACPCh. 7.6 - Prob. 2.4ACPCh. 7 - Write the electron configurations for P and CI...Ch. 7 - Write the electron configurations for Mg and Ar...Ch. 7 - Using spdf notation, write the electron...Ch. 7 - Using spdf notation, give the electron...Ch. 7 - Prob. 5PSCh. 7 - Prob. 6PSCh. 7 - Use noble gas and spdf notations to depict...Ch. 7 - The lanthanides, once called the rare earth...Ch. 7 - Prob. 9PSCh. 7 - Prob. 10PSCh. 7 - What is the maximum number of electrons that can...Ch. 7 - What is the maximum number of electrons that can...Ch. 7 - Depict the electron configuration for magnesium...Ch. 7 - Depict the electron configuration for phosphorus...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Prob. 17PSCh. 7 - Which of the following statements correctly...Ch. 7 - Prob. 19PSCh. 7 - Prob. 20PSCh. 7 - Using orbital box diagrams, depict an electron...Ch. 7 - Prob. 22PSCh. 7 - Prob. 23PSCh. 7 - Using orbital box diagrams and noble gas notation,...Ch. 7 - Manganese is found as MnO2 in deep ocean deposits....Ch. 7 - One compound found in alkaline batteries is NiOOH,...Ch. 7 - Prob. 27PSCh. 7 - Arrange the following elements in order of...Ch. 7 - Prob. 29PSCh. 7 - Prob. 30PSCh. 7 - Which of the following groups of elements is...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Compare the elements Na, Mg, O, and P. (a) Which...Ch. 7 - Compare the elements B. Al, C, and Si. (a) Which...Ch. 7 - Explain each answer briefly. (a) Place the...Ch. 7 - Explain each answer briefly. (a) Rank the...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Explain why the photoelectron spectra of hydrogen...Ch. 7 - Sketch the major features (number of peaks and...Ch. 7 - These questions are not designated as to type or...Ch. 7 - The deep blue color of sapphires comes from the...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Prob. 44GQCh. 7 - Prob. 45GQCh. 7 - Prob. 46GQCh. 7 - Which of the following is not an allowable set of...Ch. 7 - A possible excited state for the H atom has an...Ch. 7 - The magnet in the following photo is made from...Ch. 7 - Name the element corresponding to each...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Prob. 52GQCh. 7 - Answer the questions below about the elements A...Ch. 7 - Answer (he following questions about the elements...Ch. 7 - Which of the following ions are unlikely to be...Ch. 7 - Prob. 56GQCh. 7 - Answer each of the following questions: (a) Of the...Ch. 7 - Prob. 58GQCh. 7 - Prob. 59GQCh. 7 - Two elements in the second transition series (Y...Ch. 7 - Prob. 61GQCh. 7 - The configuration of an element is given here. (a)...Ch. 7 - Answer the questions below about the elements A...Ch. 7 - Answer the questions below concerning ground state...Ch. 7 - Nickel(II) formate [Ni(HCO2)2] is widely used as a...Ch. 7 - Spinets are solids with the general formula M2+...Ch. 7 - The following questions use concepts from this and...Ch. 7 - Which ions in the following list are not likely to...Ch. 7 - Answer the following questions about first...Ch. 7 - The ionization of the hydrogen atom can be...Ch. 7 - Compare the configurations below with two...Ch. 7 - Prob. 72SCQCh. 7 - Write electron configurations to show the first...Ch. 7 - Prob. 74SCQCh. 7 - (a) Explain why the sizes of atoms change when...Ch. 7 - Which of the following elements has the greatest...Ch. 7 - Prob. 77SCQCh. 7 - Prob. 78SCQCh. 7 - The energies of the orbitals in many elements have...Ch. 7 - The ionization energies for the removal of the...Ch. 7 - Using your knowledge of the trends in element...Ch. 7 - Prob. 82SCQCh. 7 - Prob. 83SCQCh. 7 - Prob. 84SCQCh. 7 - Thionyl chloride. SOCl2, is an important...Ch. 7 - Prob. 86SCQCh. 7 - Slaters rules are a way to estimate the effective...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Explain why the sizes of atoms change when proceeding across a period of the periodic table. (b) Explain why the sizes of transition metal atoms change very little across a period.arrow_forwardExplain why Al is a member of group 13 rather than group 3?arrow_forwardWrite the electron configurations for the following atoms or ions: (a) B3+ (b) O (c) Cl3+ (d) Ca2+ (e) Tiarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lanthanoids and its Position in Periodic Table - D and F Block Elements - Chemistry Class 12; Author: Ekeeda;https://www.youtube.com/watch?v=ZM04kRxm6tY;License: Standard YouTube License, CC-BY